Justice Must Be Done.
The prior election must be acknowledged as fraudulent, and steps must be taken to prosecute the fraudsters and restore integrity to the system.
Nothing else matters at this point. Talking about trying again in 2022 or 2024 is hopeless otherwise. Which is not to say one must never talk about this, but rather that one must account for this in ones planning; if fixing the fraud is not part of the plan, you have no plan.
The Audit
The Audit is definitely heating up. Let’s see if the Opposition manages to squelch it and its consequences. I’ll be honest; I expect it to be ignored by anyone capable of ordering Biden/Harris to step down.
Nevertheless, anything that can be done to make Biden look less legitimate is a worthy thing!
Lawyer Appeasement Section
OK now for the fine print.
This is the WQTH Daily Thread. You know the drill. There’s no Poltical correctness, but civility is a requirement. There are Important Guidelines, here, with an addendum on 20191110.
We have a new board – called The U Tree – where people can take each other to the woodshed without fear of censorship or moderation.
And remember Wheatie’s Rules:
1. No food fights
2. No running with scissors.
3. If you bring snacks, bring enough for everyone.
4. Zeroth rule of gun safety: Don’t let the government get your guns.
5. Rule one of gun safety: The gun is always loaded.
5a. If you actually want the gun to be loaded, like because you’re checking out a bump in the night, then it’s empty.
6. Rule two of gun safety: Never point the gun at anything you’re not willing to destroy.
7. Rule three: Keep your finger off the trigger until ready to fire.
8. Rule the fourth: Be sure of your target and what is behind it.
(Hmm a few extras seem to have crept in.)
Spot (i.e., paper) Prices
Last week:
Gold $1815.20
Silver $25.56
Platinum $1053.00
Palladium $2747.00
Rhodium $19,500.00
This week, 3PM Mountain Time, markets have closed for the weekend.
Gold $1763.90
Silver $24.48
Platinum $985.00
Palladium $2712.00
Rhodium $21,150.00
Gold was up in the 1810s all week up to Friday morning, but tanked HARD on that day, down $41.20. Everything took a beating, honestly, except rhodium which went up.
Part XIII – Rutherford On A Roll
We left off, circa 1903, having discovered radioactivity and the electron, and making quite a bit of progress with them.
To try to recap (and there are a few things in this so-called “recap” that I should have mentioned earlier, but didn’t), an electron is a negatively charged particle about 1/1830th the mass of a hydrogen atom, which up to then had been the lightest thing known to exist. They could be knocked off of atoms in a Crookes tube and they would then form what was called a cathode ray (yes, the same “cathode ray” in those big tubes in those old boxy TVs). It is possible to strip one electron off a hydrogen atom, at which point the remaining piece of the hydrogen atom (called an ion) had a positive charge that balanced the electron’s negative charge. The atom as a whole was neutral, charge 0; the individual pieces also added up to 0. Even though there was plenty of mass left in the ion, easily enough for hundreds more electrons, no one could get a second electron to come out of a hydrogen atom.
Thomson, the discoverer of the electron, suggested that atoms were fairly solid spheres of positive electrical charge with little electron inclusions that could be knocked out to ionize the atom; this was called the plum pudding model of the atom.
Radioactivity had been discovered in 1896. Uranium and thorium, it turns out, are radioactive. Radioactivity turned out to consist of three types of rays, alpha, beta, and gamma.
Alpha rays turned out to be identical to doubly-ionized helium, i.e., helium from which two electrons had been stripped (and there was no sign of being able to strip away a third electron from helium). Helium itself had been discovered on Earth back in 1895, trapped in a uranium ore; its atomic mass was four times that of hydrogen. Clearly the helium had begun as alpha particles, then combined with electrons in the ore to become helium gas. The charge of an alpha particle is 2e.
Beta rays turned out to be high-speed electrons. Their charge, of course, is –e.
Gamma rays turned out to be electromagnetic radiation, extremely strong electromagnetic radiation, like X-rays on steroids. Gamma rays, like all photons, have no electrical charge at all.
Alpha rays could be stopped by a sheet of paper. Beta rays could penetrate many sheets of paper, but would be stopped by a thin sheet of metal. Gamma rays required a lot of shielding to stop.
Uranium (atomic weight ~238) and thorium (atomic weight ~232), which had just been discovered to be radioactive, were the heaviest known elements, roughly 238 and 232 times as massive, atom for atom, as hydrogen. The Curies discovered that uranium ore was four times as radioactive as the ores it contained; they were able to isolate two new elements, radium (atomic weight 226) and polonium (atomic weight 210), by processing tons of the ore pitchblende.
It was also clear that a pure block of refined uranium would grow more radioactive over time, eventually reaching a level significantly higher than before, but not nearly as high as the ores.
In radioactive decay, the total amount of energy released, relative to the mass, turned out to be staggeringly huge, thousands if not millions of times more than what was released by burning chemicals. In 1904 Ernest Rutherford (who had named the three types of radiation, and who is the star of today’s story) suggested that radioactivity could provide enough energy to power the sun for the many millions of years necessary for Darwinian evolution to take place. (Previously known sources of energy were woefully inadequate; it was one of the 1895 mysteries I listed.)
At the time atomic weight was considered to be a defining characteristic of an element. This would cause some confusion for a few years.
Some stuff I should have covered previously, but didn’t:
The electric charge of an electron is about -1.602 x 10-19 coulombs. This is a negative number (because Benjamin Franklin arbitrarily picked one kind of charge to be positive and the other negative, and when the electron was discovered, it happened to be the one he tagged as negative), so, perhaps a bit counterintuitively, physicists define the minimum charge e to be +1.602 x 10-19 coulombs, i.e., -1 times the charge of an electron. Physicists, in fact, find it far more convenient to use e as the unit of electric charge when talking about atoms, that way they don’t have to sling 10-19s everywhere.
And they do something similar for energy. Just like a falling weight generates kinetic energy (a mass being attracted to another mass by gravity, speeds up that mass), an electron responding to one volt of electrical potential generates a certain amount of energy, which is defined to be an “electron volt.” This is abbreviated eV (which spell checkers will try to “fix” the capitalization of). This ends up being 1.602 x 10-19 joules. (Notice it’s the same factor, 1.602 x 10-19. This is a consequence of the way the joule, coulomb, and volt are defined.) Energy at the atomic level, particularly when dealing with chemical energy, tends to be a convenient, human-relatable number of electron volts.
And a reminder: An atomic mass unit was defined, in 1898, as 1/16th the mass of an oxygen molecule. This was very close to the mass of a hydrogen atom, but because oxygen reacted with more things, it was easier to use it as a yardstick. [This definition has since been modified, for reasons I’ll explain below.] It was equal to 1.6604675209 x 10-27 kilograms. (This is slightly different from today’s value.) It was abbreviated “amu.” Atomic weights were expressed in amu’s, so oxygen’s atomic weight was 16.0000, and hydrogen’s was almost exactly 1.0: In 1949, under this definition, it was measured at 1.008 amu. (At least, according to a 1951-52 CRC handbook–well, it’s a book that fits King Kong’s hand–that I happen to own.)
OK, so that, I believe, catches us up.

I’ll be honest, as I was researching this, I was surprised how many times Rutherford’s name kept coming up. I had known about a few of the things he had done (the gold foil experiment being the most famous) but in fact he was all over everything that happened, it seems. It seems he was at least in the room for a lot of things I talked about last time (like the discovery of the elctron).
He fully deserved having an element named after him (Z=104).
If parts of this caption make no sense…read on.
A Plethora of Radioactive Elements?
Scientists continued to investigate radioactivity. They would find more and more elements, distinguished by their atomic masses, in both uranium and thorium ores.
Even as early as 1900-1903 Rutherford was involved in this effort. Looking at thorium “emanations” with his student Frederick Soddy, they discovered thorium x and a gas, thoron. At first they thought these were special forms of thorium, but then they realized these were not thorium. By 1903 they had concluded that these emanations were the result of thorium changing into another element. This was a very bold conclusion, since chemists up to now had believed elements were immutable, that such things were alchemist balogna. (And under normal circumstances this was true…but radioactivity was something fundamentally new, and certainly nothing like what the alchemists had thought of.)
So perhaps these new elements could fill in the large gap between bismuth and thorium in the periodic table? Well, they could, but it turned out that in fact, there were way too many of them. Realistically between lead and uranium there was room for nine elements, and we already had five of them: bismuth, polonium, radium, radon (which was basically the thoron gas) and thorium. But just in uranium ore there seemed to be about thirty of them (based on my count looking at a chart in Wikipoo–perhaps they had found fewer than that before they figured out what was actually going on). Thorium ores brought in another ten or so.
But it was very, very difficult to separate out these putative elements. For instance Soddy in 1910 showed that mesothorium, atomic weight 228, radium, atomic weight 226, and thorium X, atomic weight 224, were impossible to separate chemically, as if they were the same element. But how could that be so when the atomic weights were different? Trying to place these elements in the table led Soddy and Kazimierz Fajans to independently come up with the notion of radioactive displacement in 1913. Basically, this stated that an alpha decay reduced an atom’s mass by about four amu (the mass of the alpha particle), and also moved it two places to the left on the periodic table. (If such a thing were to happen to (say) nickel, it would become iron, which is two spots to the left of nickel. But it won’t.) A beta decay left the mass almost unchanged (the mass of the electron that gets kicked out is relatively insignificant), but moved the element one place to the right. (If an atom of palladium were to undergo a beta decay, it would become silver. This has happened under very special circumstances, ones that won’t affect the palladium bullion I hope you own.) Gamma decay had no such effect; apparently it was just a way to get rid of energy.
For this work Rutherford won the 1908 Nobel Prize for Physics.
But he hadn’t even got started yet.
The Isotope
Now if one used the radioactive displacement principle, it appeared that two or more different “elements” could occupy the same place on the periodic table. The three I named above all fit in the same square, directly under barium. Because they occupied the same place, they were termed isotopes, from Greek for “the same place.”
So you had “elements” of different mass that otherwise behaved identically. At this point chemists decided that the mass wasn’t as important as the behavior, and swallowed the concept of two different atomic weights representing the same element, rather than insisting they must be different elements solely because of different atomic weights. Atomic weight wasn’t necessarily a crucial characteristic of an element, particularly when it came to ones extracted from radioactive ones.
In 1912, meanwhile, J. J. Thomson, who had discovered the electron in 1897 (with some help from Rutherford, it turns out) wasn’t done yet, had ionized neon (which was the tenth element listed on the periodic table at the time) in a Crookes tube and magnetically and electrically deflected its ions, the same way that he had deflected electrons in 1897, to determine the ions’ charge to mass ratio. He was quite surprised to see these ions, which should have weighed in at about 21.18 amus, went to two different locations! Some were deflecting more than others, because they were lighter than those others.
Assuming that they were singly ionized, with one electron removed (it takes a lot more energy to take the second electron off than it did the first), one group of ions had an atomic weight of almost exactly 20, the other had an atomic weight of almost exactly 22. The atomic weight of neon had been measured as 20.179, which made it one of those cases where the atomic weight was not almost a whole number, but now it looked like that was actually an average value. Most neon had atomic weight of almost exactly 20, but some came in at about 22, and the weighted (ahem) average was 20.179.
So now, even perfectly ordinary stable elements had isotopes, and this time no one thought these must be two different elements because the weights are different. In modern terms neon consists of a mix of neon-20 and neon-22.
I have mentioned in the past that many elements had “atomic weights” or “atomic masses” that were almost a perfect multiple of hydrogen’s. These mostly turn out to be elements with exactly one isotope in nature, or perhaps more than one isotope but one of them is much, much more common than the other(s).
Hydrogen, it turns out, has two isotopes found in nature, hydrogen-1 and hydrogen-2. Hydrogen-1 is overwhelmingly common, hydrogen-2 is rare, a bit more than one atom in ten thousand hydrogen atoms is hydrogen-2.
For various reasons, the isotopes of hydrogen actually ended up with “real” names–not true for any other element! Hydrogen-1 is called protium and hydrogen-2 is called deuterium.
The actual atomic mass of hydrogen is a bit higher than the atomic weight of pure protium expressed in kilograms, because the tiny amount of deuterium pulls the average up.
If, in an alternate universe, the atomic mass unit had been defined differently so that hydrogen–mixed hydrogen–got an atomic mass of 1 unit, this would actually have been slightly higher than the atomic mass of pure protium, because the occasional deuterium atom pulls the average up.
But in the real world, the atomic mass unit was defined to be 1/16th the atomic weight of oxygen. So oxygen was 16.0000 by definition. Hydrogen ended up being a hair more than 1.008. Could the excess be due to the deuterium? Not so fast. Oxygen, it turned out in 1919, consists of three isotopes. Oxygen-16 is overwhelmingly more common than oxygen-17 and oxygen-18. But even if you set pure oxygen-16’s atomic weight to 16.00 by definition, and then look at the atomic weight of pure protium, pure protium doesn’t come in at precisely 1.000. There’s still this slight tendency to be off just a bit from integers. At the time no one knew why, but they knew about it well enough to talk about a mass defect. But at least now, we understood the elements that were way off from being whole integer atomic weights–they were mixtures of isotopes. So this is a partial answer to one of our mysteries.
Physicists often discussed different isotopes of the same element. Chemists rarely did back then. Physicists used the whole number to label them, rather than the exact number. This whole number was termed the “mass number” and had the symbol A (from German Atomgewicht). I’ve been using these mass numbers a lot so far, and will continue to do so.
So we have three things with similar-sounding names. There’s the atomic mass unit (amu), almost (but not quite) equal to the mass of a hydrogen atom. There’s an atomic weight, measured in atomic mass units, which represents the mass of the atom. But there is also a mass number, which is a rounded version of the atomic weight, for a specific isotope. Hydrogen’s atomic weight is 1.0008, but the mass number of its most common isotope was just simply 1. When doing ordinary chemistry weighing out reactants the atomic weight is used to compute the number of moles of each reactant. When talking about isotopes, the mass number is used, without fail.
(Looking ahead a little: In the 1920s physicists began using a physical atomic mass unit, that really was based on oxygen-16 rather than mixed oxygen. To distinguish it from the other one, the prior one was called the chemical atomic mass unit–which the chemists kept on using. And then it turned out that oxygen obtained from water had a slightly different isotope mixture and hence real atomic weight, than oxygen extracted from the air. So the chemists’ unit was based on a foundation of quicksand. But even using the physical amu, the atomic weight of a pure isotope was still never a clean, perfect integer, except for oxygen-16.
(But now we had two slightly different units with very similar names. In 1961 they compromised, and created the “unified atomic mass unit” (symbol u, also called the dalton, symbol Da) that was 1/12th of the mass of a carbon-12 atom. This was closer to the chemists’ standard than to the physicists’.
(No matter what standard was chosen, however, the only isotope that had a perfect integer mass was the reference isotope. All others were off, just a bit.
(But that was all in the future. Let’s return to our story, back to 1912.)
The Nucleus
Backing up just a couple of years from there, there had been another very important discovery in 1909 by Ernest Rutherford. He was collaborating with Hans Geiger (who is definitely a counter) and Ernest Marsden.
They used a beam of alpha rays (which, as a reminder, are heavy and positively charged) to bombard a very thin layer of gold foil. They were pretty much expecting those alpha particles to plow through the “plum pudding” atoms. Instead, though most indeed cruised right through the gold atoms as if nothing were there, a very few of them bounced away at sharp angles, repelled by an intense and concentrated positive charge. Some even bounced back towards the beam source! Rutherford said, in a very famous quote: “It was quite the most incredible event that has ever happened to me in my life. It was almost as incredible as if you fired a 15-inch shell at a piece of tissue paper and it came back and hit you.”
In 1911 Rutherford argued that those alpha particles were bouncing off an atomic nucleus. This meant that an atom consisted almost entirely of empty space. All of that positive charge (and almost all of the mass of the atom) was in a tiny, tiny, very dense body about 1/10,000th the width of the atom; the rest of the space was the domain of the electrons, which orbited the nucleus much like planets orbit the sun, except in this case the attractive force wasn’t gravity, but the attraction between the positively charged nucleus and the negatively charged electrons. This was a new model of the atom, called the “Rutherford Model.” Rutherford is credited with discovering the atomic nucleus.
And in fact that number understates things; according to modern measurements entire atoms can be anywhere from 26,000 to 60,000 times as wide as their nuclei. Which works to to be anywhere from 17.6 – 216 trillion times the volume.
Atomic Number
Later that year, Antonius van den Broek proposed that the sequential location of each element in the periodic table was equal to its nuclear charge, this charge (in units of e) was the atom’s atomic number. This fit well for hydrogen, which could only have one electron stripped off, leaving a +1e charged nucleus behind. And for helium, which could be ionized twice leaving a +2e charged nucleus behind. They were the first and second elements listed in the table. However, we couldn’t strip every atom down to a bare nucleus to see its charge; the heavier the atom the harder it was to do that.
This was a new concept. Chemists had talked about the atomic weight of an atom, never its number. You could list the elements in the order they appeared in the periodic table, of course (accounting for the very few unfilled “holes” in the grid), but the place on the list wasn’t considered terribly significant. But now it appeared as if charges came in discrete quantities, and given that one could only remove one electron from a hydrogen atom, and two from the atom with the next higher weight, the implication was that this nucleus had a specific charge, an integer multiple of the charge of an electron (but with the opposite sign). So hydrogen’s atomic number was 1, helium’s was 2. Lithium’s was 3. And so on, through carbon (6), oxygen (8), aluminum (13), iron (26), zinc (30), rhodium (45), silver (47), tin (50), platinum (78), gold (79), lead (82), thorium (90), and uranium (92), to give some examples. (However the exact numbers for anything above the upper fifties really weren’t certain at this point.)
This was only a suggestion…until about two years later. I will pick that story up next time, because it actually ties in more with electrons, and this week I want to concentrate on the nucleus. Suffice it for now to say that van den Broek was absolutely right. I’m going to reference the concept of atomic number, abbreviated Z (from German Zahl, ‘number’), from here forward.
The Proton
So, let’s continue Rutherford’s story. In 1917 he ran some more experiments. He fired alpha beams into air (which is mostly nitrogen), and detected hydrogen ions. After refining his experiment, he realized that the alpha particles were reacting with the nitrogen. When he reported his results in 1919, he claimed that the alpha particle had simply knocked a hydrogen nucleus out of a nitrogen nucleus, reducing the nitrogen nucleus’ charge (and atomic number) and weight by one and thereby turning it into carbon. Nitrogen-14 was seemingly becoming carbon-13, a rare (but stable) isotope of carbon, which is mostly carbon-12.
But by then we had cloud chambers and could see some forms of radioactivity and ions leaving trails through the chamber. In 1925, Rutherford examined some cloud chamber tracks of this reaction, and he realized he was totally wrong about what was happening. The alpha particle wasn’t bouncing off the nitrogen nucleus after knocking one proton out of it. No, it was disappearing. What was in fact happening was the nitrogen nucleus, 7 positive charges, total mass 14, was absorbing the alpha particle.
I mentioned, up above, the principle of radioactive displacement. An atom, spitting out an alpha particle moves two places to the left on the periodic table. That means its atomic number decreases by two. The atomic mass drops by four.
Absorbing an alpha particle has exactly the opposite effect. The atomic number increases by two, and the atomic mass increases by four. So the nitrogen-14 was becoming fluorine-18.
Immediately upon becoming fluorine-18, the nucleus then shed a proton, which was the hydrogen ion that Rutherford saw. This turned it into oxygen-17, stable but uncommon (most oxygen being oxygen-16).
But in the meantime, people had decided that that hydrogen nucleus was a basic particle, and it was named the proton. It’s regarded as having been discovered in 1919, since that was the first time it was seen to exist having come from some source other than hydrogen gas. or in 1920 when someone suggested it might be an elementary particle. Rutherford, as the discoverer, got to name it.
William Prout, clear back in 1815, had suggested that the other elements might be built up, somehow, from hydrogen, and now it looked like he was at least partly right. Hydrogen indeed consisted of a single proton, mass 1, and an electron, and other elements apparently had 2, 3, 4 or more protons, all the way up to uranium with 92 of them–each with a matching electron. You couldn’t just bundle hydrogen atoms together to get other kinds of atoms, but conceivably, if you separated the electrons and protons, then combined the protons, and put the electrons back in place, you could get larger atoms.
In fact, Rutherford had suggested both the name “proton” and the name “prouton” for this particle, the latter to honor Prout. (The English would have pronounced “prouton” as if it rhymed with “grout on”, and the French would have made it rhyme with “crouton” so we dodged a bullet of linguistic confusion there.)
The proton’s mass is 1.007 amus (using the modern AMU scale). Again, maddeningly close to a whole number. But because of this, the proton looked like the underpinning for atomic number but it couldn’t be the underpinning of atomic mass. That’s because, to take an example, oxygen’s nucleus has eight protons in it, but a mass of sixteen, twice as much as the protons. Uranium is even more out of whack. It has 92 protons, but its most common isotope has a mass of 238, leaving 146 mass units unaccounted for! Why? We didn’t know, yet.
In 1920, Rutherford voiced a suggestion. He thought that the excess mass consisted of a number of protons and electron pairs, bound to each other to make a net neutral bundle. So an oxygen-16 nucleus actually contained sixteen protons, but eight of them were bundled with, and masked by, electrons. The net positive charge is eight, and that’s critical because it requires eight orbiting electrons to balance out, and those eight orbiting electrons are responsible for oxygen’s chemical properties. So the chemical nature of an atom ultimately depended on the number of protons not in these bundles.
This actually made quite a bit of sense. Remember beta decay? This is where a nucleus can spit out an electron. The electron has a single negative charge. In order to make up for that loss, the nucleus has to gain a positive charge; it’s as if a new proton were appearing. But if Rutherford’s idea were correct, rather than a proton and an electron being magically created, one of these bound pairs was breaking apart, freeing the electron and unmasking the hidden proton.
Another thing arguing in Rutherford’s favor was the fact that whatever-it-is that was left over in the nucleus had a mass that was nearly that of a whole number of protons; it would make sense for the missing ingredient to be that number of “masked” protons.
Physicists would spend the 1920s thinking that the nucleus consisted of a number of protons equal to the mass number A, plus a bunch of nuclear electrons, which left a net number of “unmasked” protons equal to Z. With some mysterious “mass defect” making the total mass slightly off.
But there were some theoretical difficulties with this…which I will take up in a future installment.
Who Cares About Isotopes?
Until late in the last century, chemists almost never concerned themselves with differing isotopes. That’s because oxygen-16’s chemical behavior is nearly indistinguishable from oxygen-17’s. Because the oxygen-17 is a bit heavier, it’s perhaps a tiny bit slower to react than oxygen-16, but not much. If you were to liquefy oxygen-16 and oxygen-17, then measure their boiling points, the oxygen-17 would require a slightly higher temperature to boil, because it would take just a little bit more energy to kick those heavier oxygen-17 atoms into vapor. Melting and boiling points are in fact the biggest difference a chemist might see…if he had separated samples to work with in the first place. And chemical means of separation were simply untenable; they were too much alike.
Water made with oxygen-17 and oxygen-18 evaporates a bit less readily than water with oxygen-16, so rainwater tends to be slightly richer in oxygen-16 than seawater (and this is part of the reason we had to stop defining the atomic mass unit as 1/16th of mixed oxygen–the mix could differ depending on where you got the oxygen from).
The chemical differences between protium (hydrogen-1) and deuterium (hydrogen-2) are actually significant, due to the fact that proportionally, the difference is greater than for any other pair of isotopes. Water made out of deuterium (“heavy water”) instead of protium actually melts at 4C, rather than 0C. I’ve seen a video of a heavy water ice cube sunk to the bottom of a glass of cold (regular) water. It’s not going to melt as long as that water is properly chilled. Note that I said the bottom of a glass of cold water. It doesn’t float because it’s heavier than regular ice and heavier even than regular water. (Now, if it were in a glass of heavy water, it would float.)
And of course, heavy water, because of its significantly different chemical behavior, is toxic when pure.
Other than that, for “traditional” chemistry, isotopes just didn’t matter.
Today things are a bit different. Mass spectrometers–which are the descendant of Crookes tubes, designed to ionize, accelerate, and deflect atoms and molecules to see how much they deflect and thus figure out the masses–are relatively cheap, and they can read out absolute numbers of “hits” at each possible mass. So one can run a sample of water through one of these and get a very precise notion of the isotopic composition. Now, you can tell whether a sample of water was rain water or ground water. Or you can analyze a sample of metal and be able to tell where it was mined, because it turns out each mine has a slightly different isotopic mix of the metal. Or one can prove that CO2 was added to champagne artificially, because the CO2 used has no carbon-14 in it (whereas the carbon dioxide in fermentation does).
Incidentally, if you’ve ever had TSA swab your luggage then stuff the swab into a machine which tells them you aren’t carrying explosives–that device is a mass spectrometer.
That’s today. But back in 1910, chemists didn’t give a rip about isotopes. Physicists studying radioactivity, on the other hand, knew that “which isotope is this?” could make all the difference in the world. And that’s even more true today too, now that we can artificially make all sorts of radioactive isotopes that don’t exist in nature. We now have to concern ourselves with radioactive hydrogen-3 (“tritium”), cesium-137, iodine-131 and strontium-90…and these were elements that were never radioactive in the days of the Model T and the Wright Flyer.
In 1910 we were just starting down this road. Remember, Rutherford had made fluorine-18 and oxygen-17 artificially.
Decay Chains
Keep this in mind as we go back now to uranium (atomic number Z=92) and thorium (Z=90). Remember that whole process of figuring out the pieces of an atom started in part because of the discovery of radioactivity, a property of these two elements in particular.
At the time of today’s story, had become quite clear that when there was radioactivity, one kind of atom was changing into another, this is called “decay.”
Uranium and thorium decay very slowly, or I should say, uranium-235, uranium-238, and thorium-232 decay very slowly (as I said, the isotope matters). It’s a statistical process. When you are looking at one uranium-235 atom, it could decay a second from now…or it could wait a billion years. There’s no way to know when it will happen, but it’s almost a stable nucleus; it’s very, very unlikely to blow in the next second. And if that atom is still around in a billion years, someone watching it then is just as unlikely to see it go kablooey in the next second as you are today.
I’m going to get on a soap box here, for just a minute. Let’s say you watch someone flip a coin 20 times and it comes up tails each time. Do you think, “wow, it’s overdue to come up heads, I’ll bet it comes up heads next time?” If so, you have a “naive” view of probability. The more sophisticated view is that, since the tosses are independent events they aren’t affected by each other. The chance is 50/50 of heads next time, no matter how many times in a row it has come up tails just now. But then, there is the cynic’s view. He doesn’t believe the odds are fifty/fifty either. But he doesn’t figure it’s overdue to come up heads; he figures the coin probably is crooked; perhaps tails on both sides! And he might have a point there. The smart bet, if you’re not allowed to examine the coin, is probably to bet on “tails.” But, if the coin really is fair, the 50/50 view is correct.
Similarly, for the chances of an unstable nucleus going kablooey in the next second, or minute. A billion years from now, provided your unstable nucleus hasn’t gone kablooey in the meantime and it’s still around, it’s just as likely to not go kablooey in the next second, as it is to not go kablooey in the next second today.
At an individual atom level, radioactivity isn’t predictable. But, if you take a large number of atoms of one of these three isotopes (or of any unstable isotope for that matter), you can make some predictions.
You can say, for instance, that any large sample of uranium-235 will be half gone in about 700 million years. Half of the atoms (no way to predict beforehand which specific ones) will have decayed to something else. Does that mean that the other half will decay in another 700 million years? Absolutely not. If you start with a pound sample of uranium-235, after 700 million years, you now have a half-pound sample of uranium-235, now mixed in with a bunch of impurities to be sure, but a half pound sample nonetheless, and half of that sample will decay in the next 700 million years.
700 million years is the half life of uranium-235. Similarly, uranium-238 has a 4.5 billion year half life, and thorium-232 comes in at 14 billion years.
You get one guess as to who discovered the concept of a half life in 1907. I’ll give you a tiny hint: He did it using one of the short-lived isotopes in the thorium decay chain, one that was deposited by decaying radon gas.
Thorium-232’s half life is about three times that of uranium-238. As you can imagine, given a godzillion uranium-238 atoms, and a godzillion thorium-232 atoms, you’ll see three times as many decays in a day from the uranium as from the thorium. But it also scales by quantity; two godzillion thorium-232 atoms will produce twice as many decays in a day as one godzillion will. And three godzillion thorium-232 atoms will produce as many decays in a day as one godzillion uranium-238 atoms. Keep this in mind–the ratio of the half lives is same as the ratio of quantity, for the same number of decays to occur from samples of two different isotopes.
[A “godzillion” is a highly technical word someone made up once for a really large number. He used it to describe the national debt when it was a lot smaller than it is now. However, even today’s national debt pales next to the number of atoms in a mole (which would be 600 sextillion or so). I decided to adapt the term rather than just say “zillions” or “jillions.”]
When an atom of (say) thorium spits out an alpha particle, it actually changes to another element and another isotope; it is decaying. If the new isotope is also unstable, it too will decay, again and again until the result is a stable nucleus. Eventually the starting thorium-232 nucleus will have become a lead-208 nucleus.
OK, with thorium being Z=90 and lead being Z=82, we can do a little bit of accounting-style sleuthing. The difference between these two masses–the change in A–is 24. That’s the equivalent of six alpha particles. In fact, since the only mode of decay that changes an atomic weight is alpha decay, we expect exactly six alpha decays to occur during this process.
But going from thorium to lead would involve changing Z by eight, which is something you’d get from four alpha decays at two apiece. Six alpha decays, absolutely required by the mass change, give you a reduction of Z by 12, and so it looks like you’d end not with lead-208 but rather platinum-208 (which if it even exists, surely isn’t stable).
Beta decays come to the rescue. They move you one element to the right, without changing the mass. So if you figure that the total number of alpha decays is six, reducing Z by 12, but then throw four beta decays into the mix, increasing Z by four, it balances; the net reduction of Z is 8. The total set of reactions boils down to:
Thorium-232 (Z=90, A=232) – 6 alphas (Z=12, A=24) – 4 betas (Z=-4, A=0) = Lead-208 (Z=82, A=208).
(Remember when subtracting the four betas, you are subtracting a negative number, which means to add the opposite positive number.)
If you look at the detailed sequence of events, this is exactly what happens. Thorium-232 decays by alpha particle to radium 228 (Z=88, A=228 one alpha decay so far). Radium-228 then undergoes a beta decay to get actinium-228 (Z=89, A=228, alpha, one beta so far). Actinium-228 undergoes another beta decay to get thorium-228 (Z=90, A=228; one alpha, two betas so far).
Let’s pause here to look at the half lives. The original thorium-232 has a fourteen billion year half life. That means that (on a percentage basis) very, very little of it decays in (say) one day. The radium-228 has a 5.7 year half life. The actinium-228 has a 6.1 hour half life. The thorium-228 has a … wait for it! … 1.9 year half life. (It’s thorium, but it’s not thorium-232 and that makes all the difference in the world when it comes to half lives.)
If you started with a pure thorium-232 sample and waited about ten years, a certain amount of radium-228 has accumulated. As it accumulates, you can detect more and more decays of it (because there is more and more of it over time. But it won’t accumulate forever: It turns out that after a few years of building up, there’s now enough of it that it’s decaying about as fast as it’s being created. So you should be able to see based on our discussion above that, given thorium-232’s half life is three billion times as long as radium-228’s, when there is one radium-228 atom for every three billion thorium-232 atoms, then they’ll both produce the same number of decays. But the radium-228 doesn’t go away, because it’s being replenished by the thorium-232 decays. Since the amount isn’t changing over time the radium-228 is in equilibrium with the thorium-232. (The thorium-232 is slowly going away, of course, as it does so it will produce slightly less radium-228 during a given time, so the radium-228 will decline at the same percentage rate. But people don’t live long enough to see this happen, not with a 14 billion year half life!) Equilibrium is reached in something like 1 1/2 or two half lives of the daughter isotope.
Similarly for the actinium-228–because it has a much shorter half life than radium-228, it reaches equilibrium with the radium-228 almost instantly. And so on down the chain. Once everything is at equilibrium, there is one decay of each daughter isotope, for each decay of a thorium-232 atom. This is why a “pure” sample of thorium actually grows more radioactive right after it’s made.
So back to that chain. It continues. Thorium-228 alpha decays to radium-224 (Z=88, A=224, two alphas, two betas so far). Radium-224 alpha decays to radon-220 (Z=86, A=220, three alphas, two betas so far). Radon-220 alpha decays to polonium-216 (Z=84, A=216, four alphas, two betas so far). Polonium-216 alpha decays to lead-212 (Z=82, A=212, now five alphas and two betas so far).
Lead-212 is lead, and lead dug out of the ground is stable, but lead-212 is not stable. It’s an unstable isotope, a very unstable one in fact. Its half life is 10.6 minutes.
The next step is a beta decay, lead-212 becomes bismuth-212 (Z=93, A=212, five alphas, three betas). We now have just one alpha and one beta decay left to get to lead-208. But now, the path splits. We can either do the alpha decay first then the beta decay (thallium-208 (Z=81), then lead-208) or the other way round (polonium-212 (Z=84), then lead-208).
All of these decays from thorium-228 onwards have half lives of days or less, one even has a half life of less than a millionth of a second. So once the thorium-228 reaches equilibrium with its great-grandparent thorium-232, the rest of the chain ends up in equilibrium in just a few days.
The diagram below summarizes this whole process. And it uses a notation I haven’t used yet. So far when I’ve named an isotope, I’ve done it as [element name]-[mass number]. But you can also use a superscript before the element symbol like this: 232Th. Superscripting is a bit of a pain in the ass in the WordPus editor (and besides you might not know all the symbols), so I didn’t do it this way. It can even be taken a step further (and is, in the diagram below). You can put the atomic number Z as a subscript before the symbol, like this: 90Th. (Or you can do both. And I do mean you can do both. I can’t. If I try, I get something like this: 23290Th. I can’t get the super and subscripts one over the other.)
Technically the atomic number is superfluous, thorium is by definition atomic number Z=90. But it’s helpful for all the non-geeks out there who don’t have the numbers memorized.

(Even chemists don’t usually know all of the atomic numbers, nor do they know all of the symbols; I watched one give a lecture on this very sort of thing, and when he showed the symbol Pa, he called it “palladium” (it’s actually protactinium, atomic number Z=91; palladium’s symbol is Pd and its atomic number is Z=46 and its price is almost three thousand dollars an ounce. The symbol was right, his verbal reading was wrong). Chemists will know the common elements like sulfur (16, S), plus ones they themselves are personally working with…unless they’re complete geeks, in which case they’ve memorized them all. By the way, if you ever run into someone claiming to be an organic chemist and they don’t know that carbon’s atomic number is Z=6, he’s a faker. Actually, he’s a lying sack of bearded dragon shit. Run, do not walk, away, from this person, and do not believe him if he tells you that the sky is blue; don’t even believe him if he says that Joe Biden lost.)
One last thing to note about the thorium decay series. Every single isotope on it has a mass number A that divides by four. The starting number divides by four, and any time the mass number changes, it changes by four, so it will always be divisible by four.
The other two decay series have uranium in them. Uranium has two long-lived isotopes, and they are each at the beginning of their own decay chains. You can walk through them if you so desire, but I’m just going to put up the diagrams. The first is the “Uranium decay series” starting with uranium-238:

Every one of these isotopes’ mass numbers, when divided by four, leaves a remainder of 2. Therefore, none of these isotopes appears in the thorium decay series, and none of these appear there either. Never the twain shall meet.
Note that one of the intermediates is uranium-234 with 245,000 year half life. If you (personally) start out with pure uranium-238, you won’t live long enough to see it come into equilibrium with its daughter isotopes, because uranium-234 decays too slowly. Over about the next half million years, 234U will build up in the sample and then be in equilibrium. Everything downstream from it is much faster. You will see, rather quickly, the intermediate thorium and protactinium 234 isotopes reach equilibrium, though.
The uranium-235 series is actually called the “actinium decay series” to avoid confusion with the other uranium decay series. It includes the longest-lived actinium isotope, actinium-227.

All of these isotope mass numbers, when divided by four, leave a remainder of 3. They therefore won’t appear in either of the first two series, or vice versa.
There ought to be a fourth series, one where all the mass numbers leave a remainder of one when divided by four. Right?
Well, there was. A long time ago. The problem is no isotope in that series (which we can reconstruct today since we can make artificial isotopes) has more than a 2,140,000 year half life. That’s much shorter than the uranium and thorium isotopes in the other series. That isotope is neptunium-237 (Z=93). One of its daughters is uranium-233, with a half life of 159,200 years. Everything else in that series is shorter, much shorter.
If there was any neptunium-237 on earth when it first formed, ten half lives (21.4 million years) would have reduced it to 1/1024th of its original amount. Another ten half lives would have reduced it to less than a thousandth of a thousandth, or less than a millionth of the original amount. A total of eighty half lives would be enough to reduce an entire mole of neptunium to less than one atom on average, an undetectably small concentration, especially since the neptunium probably started out as a minor constituent of whatever rock it was in, to begin with. (Realistically, fifty half lives is probably enough to escape detection by modern equipment.) Seventy half lives is about 170 million years.
There was either never any neptunium-237 when the earth formed, or the earth is at least 170 million years old. In fact, there are a lot of isotopes with even longer half lives (like plutonium-244, half life roughly 80 million years) that do not exist in nature, and the same logic applies: either that isotope was never around, or the earth is hundreds of millions of years old, or even older–plutonium-244’s absence implies billions of years.
Returning to the “neptunium decay series,” because it has no sufficiently long lived isotope, it is extinct. When we started making isotopes artificially, we eventually found neptunium-237, and uranium-233, and all the others, and could then figure out what the neptunium decay series looked like. But back in the 1910s, this was all well in the future.
[Actually, oddball nuclear reactions sometimes create a trace of these isotopes in uranium ore, but that’s an almost immeasurable trace, and clearly not remnants of an original stock.]
The second to last product of the neptunium decay series is bismuth-209. It was long thought to be a stable isotope, but fairly recently it was discovered to have a half life of 19 quintillion years-almost a million years for every dollar of our national debt. It is so weakly radioactive that it might as well be stable, and its radioactivity is consequently almost impossible to measure. When it bestirs itself to do so, it decays to thallium-205, which is unfortunately quite stable. I say unfortunately, because thallium is extremely toxic. There is actually plenty of thallium-205 out there already, but it has to almost all be original or primordial stock, because hardly any bismuth-209 has decayed in a mere few billions of years.
Summing it up
Radioactivity was discovered in 1896. At that time, the words electron and proton didn’t exist. Atoms were indivisible things. Twenty years later, we knew that last bit was wrong, and we were well on our way to knowing the real nature of matter. In large part thanks to Ernest Rutherford.
OK. Next time, we take one step out, back into the realm of the electrons.
Obligatory PSAs and Reminders
China is Lower than Whale Shit
Remember Hong Kong!!!
中国是个混蛋 !!!
Zhōngguò shì gè hùndàn !!!
China is asshoe !!!
China is in the White House
Since Wednesday, January 20 at Noon EST, the bought-and-paid for His Fraudulency Joseph Biden has been in the White House. It’s as good as having China in the Oval Office.
Joe Biden is Asshoe
China is in the White House, because Joe Biden is in the White House, and Joe Biden is identically equal to China. China is Asshoe. Therefore, Joe Biden is Asshoe.
But of course the much more important thing to realize:
Joe Biden Didn’t Win
乔*拜登没赢 !!!
Qiáo Bài dēng méi yíng !!!
Joe Biden didn’t win !!!

I debated bringing up the Chris Chan story here because this story is a soap opera straight from the depths of hell. But now even Tucker is talking about it, so I guess it’s fair game. I’m warning you all, though, if you don’t want to read about people being sadistic and horrible to each other, then keep scrolling. Seriously, this is “incomprehensible evil” levels of bad. The only reason I’m bringing it up here is because the whole thing is starting to glow.
For the uninitiated, Chris is a severely mentally ill internet celebrity. He rose to fame over the past 20 years due to his cringey, narcissistic behavior, and being targeted by sadistic trolls. There’s an entire Wikipedia-like website about him, and a 60+ hour Youtube documentary if anyone wants the backstory. It was a morbidly fascinating rabbit hole up until last week. Then it got incredibly disturbing.
Last week, Chris Chan admitted in a recorded phone call to having a sexual relationship with his mother. His mother is a frail 80 year-old who very likely has dementia. The police were called, the mother was taken to the hospital, and Chris was arrested. He’s currently in jail awaiting trial without bail.
This is what the Tucker segment above is about. Chris decided to become transgender a few years ago because he thought it would help him attract women. The jail booked him as “female,” and now it’s possible that the man who raped his mother could very well end up in prison (if convicted) with other women. Peak Clown World stuff.
Sadly, this story gets even more screwed up from here.
The person on the other end of that incriminating phone call is a young woman named Isabella Loretta Janke. People started digging as soon as the horrible news came out. She’s a real piece of work who introduced Chris to various occult stuff, and has a documented history of evil behavior that I won’t speak of here. It turns out that Isabella had a grand plan to encourage Chris to commit incest, blackmail him (hence the recorded phone call), and then ultimately encourage a murder-suicide of Chris and the mother.
I told you this was bad. Here’s where it starts to glow.
Isabella’s father is Michael Anthony Janke. Mike Janke is a former member of Seal Team 6 who now owns a bunch of military contracting spook companies involving both tech and Hollywood. Isabella’s mother is Mary Beth Janke. She’s an ex-Secret Service agent turned clinical psychologist.
(Source all of all this info)
Prior to the Tucker segment, I had wondered why a case this sensational hadn’t hit the tabloids and media. At first I thought it was because they were trying to protect the overall tranny agenda, and they knew how bad this would look. But upon learning of the spook connections, I suspect the real goal was to publicly distance the glowies and agencies as far from this sordid story as possible. Maybe Mike Janke used his tech and media connections to call in some favors to protect his horrible daughter. Maybe the cabal doesn’t want the public speculating on what kind of sick people are connected to the glowies.
Maybe Chris Chan himself is some three letter MKULTRA-experiment (remember, Mary Beth Janke is a clinical psychologist). Maybe he’s a case study for weaponized internet trolling. I don’t know, but I don’t believe in coincidences.
Anyways, the reason why I decided to post this is because we’re all familiar with the two-tier system of justice in this country. Isabella’s parents will use their wealth and deep spook connections to make sure Isabella never sees any real punishment for her horrible behavior. Hell, they’ll probably hook her up with a well-paid spook job in DC since she’d fit right in with the rest of the sociopaths running DC. The only justice that will ever come out of this is through shining a light on this horrible person, and making sure she never harms anyone else.
What’s a glowie?
Wow, I had heard the headline version, but didn’t know all this. I admit, my antennae started to twitch when I got to the spook part. Thanks Sadie.
I vaguely recall that it’s someone deeply Fed trying to pass themselves off as normal.
Thanks coothie!
Well, close….
“Glowie” is an insult for intelligence agents. “Glow” and “glowing” means that there’s undercover CIA or FBI-type involvement. The origin of this term comes from the Terry A. Davis video linked below where he talks about “glowing CIA niggers.” That particular insult is still common on the chans, but “glowie” is the much more socially acceptable version for polite company. Terry was an eccentric man who believed using offensive language was a defense mechanism against psychological warfare. I do not believe he had racist intent when he made this video.
Warning: Extreme language
Bonus: Look at the computer screen in Terry’s video: “China Virus Election.” This was recorded in 2017.
Thank you Sadie. I appreciate it.
The “glowie” term is mostly used by the anons when they discover a plant / infiltrator (most times almost right away).
It’s like saying to a mal-poster that he’s so OBVIOUS that he “glows”.
“You glow” = “I SEE YOU” (for what “you” are – a subverter).
Clown says: “I’m an anon just like you!”
Anon replies: “Nah – you glow! You’re busted, glowie!”
And the undercover clown is outed – he’s blown his “cover” – and his disinformation attempt fails.
I’m at a loss for words. The evil in this world is rampant.
The presence of incest, occult, spooks, and generations in two families, tells me that this is definitely MKULTRA / demonic stuff spilling out into the normie world.
Now – here is a tip on something that the anons may not have discovered yet.
Based on what I’m seeing here, there is extreme likelihood that Chris Chan’s mother is also an MKULTRA subject. Thus, people researching her past need to look for the usual signs.
Listen to Isabella’s father here:
His early association with privacy encryption pioneers makes a lot of sense. IMO a military MK subject. Mom likely, too.
But where all that goes, I have no clue. There are a lot of possibilities.
Oh definitely. The mother has a long history of being abusive, manipulative, and, up until last week, was widely considered the least sympathetic “character” of the whole story. The incest rumors were around for years. There’s also a weird gap in Chris’ childhood where she lived alone in northern Virginia (literally spook central) away from the family. MK victim is certainly possible.
Thanks for that info!
Check out Isabella’s mom. Three Banners and NPR. Very clear which side of this sh*tshow she’s on.


Clinton-era Haiti connections, too.
https://blog.darlingmagazine.org/mary-beth-janke-interview/
Oh yeah, and her current occupation:
Thanks! Fascinating. THAT explains a LOT.
Beelzebubba & Hellary
So reading between the lines……. possible grooming grounds for, as needs arise for subjects to act on given orders??
Yes – I think there is some connection here. I’m not sure what it is, exactly, but I think you may be onto larger purposes.
Looking at her, I get the spidey-sense creepy feeling like when I see an OT-level scieno, or a Satanist witch (not as much difference between the two as many would think, cf. history of L.Ron Hubbard).
And something seems off about her arm positioning… sort of the feeling one gets from seeing a guy with his hand tucked in his vest like Napoleon and others did…
Also thinking back to a few years back when the Klowns in Action were going to flood the election with their own candidates… No-one seems to talk about that anymore, but I wonder who they were/are…
>>”And something seems off about her arm positioning”<<
Crossing ones arms is usually indicative of DEFIANCE.
An “F U” … or even a “F EVERYBODY”.
Good intuition, Cuppa!
I agree.
Her right arm is longer than her left arm. Compare the 2 upper arms down to the elbow. The right arm is longer.
Cuppa, I scrolled up to check out what you mentioned about her/? .. crossed and when I made the picture bigger her head does not fit her neck. It’s seriously creepy …
.. 😳 .. 😖 .. 😳 ..
Something isn’t right about the picture or her. Maybe she’s a guy I don’t know.
Now I have to go find the Pepto Bismarck .. yuk 🤢
…when I made the picture bigger her head does not fit her neck
^^^ Pencil Neck’s sister?
Pencil Neck’s mister…
The sinking of the Bismol 🙂 or 
… bwahahahahaha … lol … 

 ..
..
well … three’s a good number ..
Big fingers/ hand………for a woman
Isn’t Jean Bertrand Aristide the Haitian president known for child sacrifices?
This Aristide? And what about Marina Abramovic… who looks sort of like this woman… also a Satanist… Seems wackypaedia thinks Aristide is now a Catholic Priest (Salesian order)…
https://www.coreysdigs.com/haiti/clintons-haitian-black-magic-secret
Cuttingedge has tons of info on the Klintoons and their Satanic Black Magick Illuminised Witchcraft… The book “Unlimited Access” by Gary Aldrich has a lot of background material as well (e.g. the Clinton Christmas Tree)…
WOW, that’s quite the link. Clintons visited Haiti and alleged participated in a voodoo ritual on March 31, 1995. That’s the same year Mary Beth Janke claims she was in Haiti protecting Aristide.
OMG. OMG. OMG.
Also:
The entire article is well worth a read.
People don’t understand the powers at work here. So many look at Ephesians 6:10-18 and just kind of gloss over it.
The Devil doesn’t. Nor do his demons. Nor do the DEMONRATS.
They seek every which (witch?) way to do their dirties, and hide all traces, and make it look like the “good guys dunnit”…
We must be ever vigilant, ever prayerful, and ever at ready to fight the wicked ones WITH GOD’S HELP, STRENGTH, GUIDANCE, AND PROTECTION.
For if we do not stand, they will win.
And here’s a bit more. Consider that the Klintoons had access to ALL FBI, Klowns-in-Action, and NSA records since the Billary “administration” began; it’s pretty obvious that they had the goods on everyone in DC, and probably STILL DO…
Corey has dug quite deeply here. And there’s much, much more at other, parallel sources that confirm this. And the MASSIVE evil, from the pit of Hell that accompanies and “guides” all that is Clinton, lives on in the Clinton Foundation, the DEMONRAT party, the Øbøzøs, et. al. , and the demonic world of the globalists, including Big Pharma.
Again, we need prayer. MUCH prayer, and fasting.
I heard something like this before but not in such detail. What is so heart wrenching that these people gained so much power.
Yes we have to pray and pray hard the Lords Prayer for protection. We are fighting in a spiritual field and God’s light will overcome the darkness. We are God’s soldiers.
With God all things are possible.
I suspect this is happening right now at Obama’s “birthday party.” August 8th = 8/8. They’re probably hoping a ritual will save their sorry butts from the incoming election audit news.
-sigh-
May God send them into confusion, cause great divisions amongst them, disempower them, demolish every structure, destroy every alliance, dismantle every purpose. May His presence fill every place they run to so that they find no rest or ease for their mind.
AMEN!
AMEN!
And may Almighty GOD take down, disempower, thwart, and utterly destroy the Devil’s thrones, dominions, principalities, powers, and demons of every kind that are controlling and enabling the DEMONRATS, the globalists, the RINOs, and all who are acting to corrupt GOD’s Will, His Creation, and His people.
BTW, Cuppa, there’s a German internet celebrity named Drachenlord who is considered the “German Chris Chan.” I don’t know much about him because of the language barrier, but head’s up if he starts appearing in tabloid headlines or weird German scandals.
https://knowyourmeme.com/memes/people/drachenlord
Thanks, Sadie, I’ll have a look. There are lots of evil folks over here, and not just leftovers from Hitler’s “coven”. The Greens have a very VERY dark side (back through Rudolf Steiner and the earth worshippers, etc.)…
Now they want to tear out thousands if not millions of acres of trees to plant birdchoppers, I guess to help worship their goddess GAIA.
G reens
A re
I diots
A lways
(Also Hilly and Billy give me the willies. They must have a TON of information.
I wonder whatever happened to those two planefulls of Bankers’ Boxes that left Arkansas for parts unknown???)…
Apple plans to scan U.S. iPhones for child abuse imagery. Stephanie Hamill, a reporterette on OAN hosts a show where she interviews people. She was talking to a guy last night who lobbied the government on issues of sex trafficking, and she offhandedly said something like, “We don’t actually hear much about this issue, but I assume that is because it is bipartisan, and there is no argument over it.” The guy immediately recoiled and responded, saying roughly, “Oh there is a massive disagreement over it, and it is immensely partisan. We have been lobbying for specific actions for 20 years on sex trafficking, and in just four years, through executive action, President Trump enacted everything we had asked for. And already the Biden administration has undone all of those programs. And they have even defunded the Dallas PD’s Vice unit. There is no Vice Unit in Dallas!” I have wondered if US Marshals were always rescuing kids, and we only heard about it under Trump, but apparently not. The massive reduction in all of those stories about kids being rescued apparently is reflective of an underlying reality – the Democrats are protecting the traffickers and Trump was taking them out.
A man captured on video during a child predator sting operation claimed to work for House Speaker Nancy Pelosi, Hillary Clinton, and other high profile Democrats, and appears to have been photographed alongside prominent Democrats including Rep. Sheila Jackson-Lee and Chasten Buttigieg, the husband of Biden administration Transportation Secretary Pete Buttigieg.
…  … 😔🙏🏼 … 😾 … ‼️
… 😔🙏🏼 … 😾 … ‼️
Chris Chan, 39, is listed as female in the police report and will be jailed with women inmates despite his ‘arrest for raping her dementia sufferer mother, 79.’ Here is the weird thing about this. They say because Chan identifies as female, and the law says the incest he was arrested for has to be between a male and a female, Chan’s defense will be he identifies as female so the law is unimportant, and the case will probably go to the Supreme Court and be precedent setting.
It’s such a crazy story that it wouldn’t surprise me if it ends up becoming some landmark Supreme Court case about trans rights.
https://twitter.com/KeithWoodsYT/status/1421804885109100544
I had not encountered this story before, and, seriously, the truth is stranger than fiction.
Occam’s razor fails in the light of reality when it comes to the forces of darkness walking this earth.
Truth is always stranger than fiction, which makes me wonder why people are so addicted to fictional drsma.
Why watch “the West Wing”, for example, when there is no way Hollywood writers could dream up what happens in real life?
This is a rhetorical question, btw.
I gave up night time dramas in the 80s.
I used to read a LOT of science fiction. After 9/11/2001, I stopped. Who needed fiction after that? Oh wait… I did get Catturd’s Rabbitskin and enjoyed it a lot. hehe
Her parents are into bad crap too it seems. Not a chance they are unaware.
Still trying to dig up part II but this is good enough.
Someone needs to hack into Fox news and run this video 24/7. Wow!
Yes, and by this guys standards we are very well informed.
Yes! Kudos, QTreepers!
And airports, doctors’ and dentists’ offices and gas pumps everywhere…
Take that, SHELL!!! 🙂 Get the lead out and do something about it…
Thanks for this. Hope Part 2 shows up.
Hoping this will play here…Click on the video below the first pic.
https://twitter.com/HoutmanBas/status/1423824794945003521
This guy is great.
It’s sublime.
.
And this is most of us.
https://twitter.com/AlexGiorgio6/status/1423789131214180355?s=20
Rochelle Walensky and Tony Fauci have lied a few too many times for me to take certain vaccines again. I’ve never missed a flu vaccine, but I no longer trust these people. Not sure I’m an anti-vaxxer, but these liars appear sufficiently desperate to muck with the flu vaccines, so I’m holding off until CDC is cleaned out with a firehose or hell freezes over, whichever happens first.
Regarding, “until CDC is cleaned out with a firehose” — suggest “until CDC is cleansed
outwithafirehose“.LOL!
Officially declaring as an anti-vaccer. Their whole premise is they solved polio. Since then they have a vax for everything, even though their data does not support it. Heal thyself.
The one I always hear is whooping cough since my mother had it as a baby and it scared her parents to death.
The whooping cough vaccine. THAT was one where we experienced vaccine breakthrough in our family. But NOT in my dear mom, who like yours had the disease as a child.
Yes, very scary for parents. And my babysitter who had smallpox told me all about people dying of diphtheria. Her descriptions were rather terrifying. I think she said something to the effect of “at least smallpox didn’t strangle ya”.
Yes. I’m definitely not anti-vax. I’m anti-CDC-corruption.
WOLFIE<
I think this guy THE MARKET TICKER sums it up:
There are two types of ‘Jabs’
A sterilizing vaccine that prevent infection
AND
A JAB that permits the virus to infect you and replicate BUT DOES NOT GIVE YOU SYMPTOMS aka prophylactic therapy.
The JAB IS DESIGNED TO SPREAD THE DESEASE, PROMOTE MUTATIONS AND PROMOTE ‘VACCINE ESCAPE’ aka virus immunity TO the ‘prophylactic therapy.’
This is what Dr Malone, inventor of mRNA technology was talking about when he said “YOU DO NOT USE THESE VACCINES IN THE HEAT OF A PANDEMIC!!!”
That is what Geert Vanden Bossche said, too.
It is our DUTY TO SCIENCE to reject these vaccines.
I had diphtheria when I was 4 and lived to talk about it.
Yikes!!! Very happy you made it through. That’s ROUGH.
Not really. I was in a Catholic Hospital full of nuns they spoiled me and I got to eat they had food 🙂
Was taking a class some years ago and we were all in close quarters for the semester. One of the students was in military and had a young child. Child was given the whooping cough vaxx. Both child and parent got horribly sick. No one else in the entire class was sick.
YIKES.
Jonas Salk is probably rolling in his grave over this.
The polio vaccine was his life’s work, and he wasn’t a corporate shill with billions of dollars and unlimited political favors coming his way.
No, today’s crop of of corporate leeches aren’t worthy of cleaning out his wastebasket and washing his lab equipment.
They aren’t worthy of existence at all…
The CDC needs to be purified. Maybe with fire. Dismantling might not be enough.
We need a FLOTUS named Melania to do an exorcism.
Or hire an exorcist.
BOTH, please!!! 😀
FIRE PURIFIES!
REMEMBER:
The CDC Foundation is an independent nonprofit and the sole entity created by Congress to mobilize philanthropic and private-sector resources to support the Centers for Disease Control and Prevention’s critical health protection work.
AND
Our Partners: Corporations
A whose who of Big Pharma, banks and even Starbucks
Our Partners: Foundations (a quick scan shows)Bill & Melinda Gates Foundation
Blue Cross and Blue Shield of North Carolina Foundation — HMmmm
Foundation to Promote Open Society aka Soros
The Rockefeller Foundation
Rothschild Revocable Living Trust
and a ton more such as
Our Partners: Organizations
That is where you find THE HOSPITALS AND UNIVERSITIES!
OMG. CDC was taken over.
That’s why this picture:

You completely described me as well in that post, Wolf. Traditional vaccinations typically add good value to the human immunity system. None of this mess can be described in that manner. We have no clue what will be in the flu vax as they have proven to be extraordinary liars and generally evil people.
Exactly! And even more, “flu communism” is every bit as bad as “covid communism”. I want NEITHER!!!
TradeBait, I read somewhere yesterday that Moderna has a combo flu shot in early Clinical Trials.
Ask your Doctor if you are one to take a flu shot!!!
There is zero chance of my taking the flu vaccine.
In addition to CDC, FDA, NIH and all them lying bastards lying to us daily, Big Pharma is creating flu shots that include Covid crap.
AND, we have no idea what they put in the flu vaccines.
NO LONGER TRUST ANYTHING CDC, FDA, NIH…related.
Hmm. Now that there seems to be a shot they really, really want me to take but I refuse to take, I’m sure I don’t want ANYTHING. YOU, after all, can’t tell what’s in that vial. They don’t show you the label, and at this point I wouldn’t trust the label.
More vile than vial…
Yup. Those combined vaccines are an affront to mankind.
Medical mafia, out of control.
… evil …
The beings in that photo are pond scum, they’re facing judgment, eternity .. they do not prevail against God even with all the horrific evil they commit … they’re … mortal and will die however their souls ….
Cue to 6:49 🔼 ..
Hmmm.
“Take this jab and shove it,
I ain’t followin’ you no more”…
comes to mind…
Something that really frustrates me is, why didn’t we all see this before?
There were authors like Vance Packard (“The Waste Makers”, “The Naked Society”, “The Hidden Persuaders”, and many others) who were sounding a clarion call about big biz and the elite BACK IN THE 1950s!
Yet no-one listened…
I hope and pray that we all can set things right, with GOD’s Help, Power, and Guidance.
Otherwise it seems that all that’s left is to burn the bl00dy thing down and rebuild it from scratch.
Maybe the Perseids will help out, and take out Brussels, Davos, Berlin, Strasbourg, Davos, wherever Sauros and Schwab are, DC, Peking, and other dens of iniquity…
I was watching a YT channel I haven’t visited in a while. A pediatrician in Oregon. He was disturbed by the movie “Vaxed” and so did a study among his own patients comparing the kids who got the usual schedule to the ones who didn’t and published the results. The kids whose parents chose NOT to have them vaccinated were, in general, healthier. His mistake was publishing that study. His license was suspended. He’s back practicing now.
Anti-vaxxers not only trust their immune systems, but they’ve done their homework.
Yeah, that’s interesting, isn’t it?
My daughter, who vaccinated her children, but back before the current aggressive vaccine schedule, will no longer vaccinate them.
She told me that she knows both unvaccinated and vaccinated toddlers, and that the vaccinated ones are sluggish, slow, and not as bright. I believe her.
My one nephew, I think, is vaccine injured. He’s BRILLIANT, but has a number of issues including developmental delays. The current diagnosis has to do with his mother not being able to absorb B12, but that doesn’t explain the ADHD. This kid was also 7 weeks early. So, all of it, I’m sure, contributes.
I’ve seen this as well. Children of neighbors that were extremely social and precocious and then they were not.
Yes. It is so sad.
We got significant amounts of smoke on Friday. It’s supposed to clear out by Monday.
EVERY TIME I hear them say, “this area of brush hasn’t burned for 100 years”, I can feel my heart pounding inside my chest. Idiots.
Yeah, I hear you. This endless drought is putting us all in another dangerous situation like we had last year. They must have HAARP turned up to eleventy.
The dangerous situation is the “hasn’t burned in 100 years”. If they’d been intelligently managing the forests, rather than having been environmental goofballs for the last couple of generations, the worst they could say was, “this area has 20 years of accumulated brush and was on the schedule for next year. It should still be pretty safe, however, because it is surrounded on all sides by more cleared areas.”
My Chiropractor’s wife’s family founded a Town in CA that burned to the ground this wk.
Firefighters were told to report back to base, and just let it burn.
Wonder how often this little detail is omitted?
The rot is deep.
Is that that “ghost town” I was reading about?
wolfie, no…people were living there. kalbo asked for name of Town. I’ll call Monday for Town name. She is devastated. she grew up there.
Wow.
IF you don’t mind, the town is?
Kalbo! C’mon, man!
You’re asking for a partial doxx.
Curiosity ****ed the cat?
YIKES!
Seriously?
Perhaps this is another confirmation that I’m much slower, than I thought. Gotta ponder ever posting like questions, which I at least superficially do.
Not asking anyone to dox themselves. The answer could be incredibly obscure from linking THE town that burned THIS wk, to THE individual. Only the poster knows that answer.
It’s the Chiropractor’s family that founded the town. I assume it is not the poster’s family, also. Dunno.
Since posting I quit being lazy and Googled a town that burned down this wk. At least one town readily available via Google.
My asking the town is IMO, no more doxing than the poster stating, Chiropractor’s relatives founded a town that JUST burned this wk.
^^^ THIS, IS, where I was coming from. IF the answer is harmless to poster, great. Share or not. Potentially harmful, as in potential dox, NO answer is obviously the way to go.
—-
What would be of far more interest to me, is the government official’s name that ordered fire fighters to abandon efforts to save the town structures.
—
On that happy note, back to the Keurig for a second cup of coffee. 🙂
You might look to ThinkGeek for more ways to use your Keurig 😆
kalbo…I can’t remember. Monday I’ll call and ask. Will put my response in notifications.
kalbo…the name of the Town is Greenville.
Thanks. Sad what has happened there. Beautiful area.
The ALSO got rid of the fire roads and FIRE BREAKS!!!!
Prehistoric Indians KNEW BETTER! They intentionally burned to clear out the crap and PROVIDE FOOD FOR THE CRITTERS THEY HUNTED.
https://sharylattkisson.com/2021/08/forum-whose-information-do-you-trust/
“Yuri, you are always reading Pravda. You can never know the truth from reading that, so why do you keep reading it all the time?”
“Sergey, I don’t know what the truth is. But I know if I read Pravada, I’ll know what lies they want to trick me into believing.”
A group of honest people, working together, can often find kernels of truth among bushels of lies.
I trust US, Michael.
We here have been right about almost all of the covid information from the beginning.
I can’t possibly live the life I do and read everything I need to, see everything I should, and discern what to do. But I know that somebody here, and the list is too long to write, will have seen it, read it, and put it here.
So I read here, every comment, every day.
I think it might just save lives, including my own.
I feel the same way, Aubergine.
There’s a lotta talent here, and it’s all guided by getting to the TRUTH (come what may).
We’re the way “diversity” WORKS – diversity of perspectives, knowledge, wisdom, and other gifts – yet we’re laser-focused on this mission: for truth, for family, for country, and above all for GOD.
Despite the inevitable disagreements – which are predominantly MINOR – we have a unity of PURPOSE.
And we exercise FREE SPEECH *every* day.
Like you (and others, yes?) … I find solace here.
Well said, total agreement.
Yep, and you know what else? I DON’T KNOW what COLOR you are, and I don’t care! DIVERSITY, the way it COUNTS!
True blue.
CAREFUL There are BLUE HUMANS! 🤓😜🙃
The Blue People of Kentucky
http://www.messagetoeagle.com/wp-content/uploads/2019/03/bluepeopleofkentucky.jpg
We do, however revel in our purple people.
Lol!
Purple people are people, too!
PLM — Purple Lives Matter.
LOLOLOL!!!!!
Pronounced “PLUM”, according to the Professor who runs it (from the Conservatory).
B I N G O !
My days start with reading QTree. Visit QTree several times everyday. Smart honest folks here. Superb spread of education, professional / life experience and wisdom garnered over the decades..
Best I can tell all dedicated Patriots. My kinda place. 🙂
Mine, too!
On a happier subject, I’ve just initiated a one-hour transfer of data files that will hopefully make the Fiancee happy tomorrow. She’s kicked, screamed, dragged her feet, obstructed, and complained for years about Linux for years (while missing out on all the people shoved rudely off of Win7 onto subsequent malware)…..and I have high hopes that things should save her bacon at the end of this.
It’s kind of a special case, in that half of what she deals with daily is her open tabs and browser bookmarks, and I’ve found the way to preserve them.
And data transferred, system shut down….
Tomorrrow, I get to see how well I can reload….
You ever look into dealing with the security issues from USB keyboards? I just ran across some writing on it and it felt like the ground was crumbling under me.
USB anything is a security nightmare. The protocol is completely fargleschnitzeled — Host: “Hello, new USB device — tell me how to deal with you.” New USB device: “First, ignore security; second, run this ‘*cough* driver’ that will initialize the device, access a malware server in a foreign country, and start downloading malware; third, accept any data from this device as valid without running any sanity checks……”
The protocol in real secure systems is to just fill the sockets with epoxy.
Qubes has a complete subsystem for USB that can’t touch anything else.
But if you have no reason to believe you’re being personally targeted, you can probably use generic USB hardware that was never specifically meant for you (e.g. a USB keyboard from a surplus store) without worry.
So Qubes is firewalling it a little bit? I’d rather use something other type of connection, but I am rather fond of my current keyboard and track ball. I do know of someone that needed a non-usb solution and did some kind of conversion to fiber optic. Perhaps an intermediary box that only does USB keyboard handling would be nice. Right now I physically unplug keyboard/mouse from one system to plug into another system. A smarter/secure KM switch would be worth finding.
If you’re on Linux, everything’s a file….any keyboard, mouse and display for a computer can be any keyboard, mouse, and display on a network.
Would you be kind enough to point me at some educational pages on this? Feeling a bit slow.
Please ask earlier in the day about 10+ hours from now.
I’m about to fall asleep and am liable to spout gibberish.
The Qubes concept is to take a bare-metal hypervisor and isolate every part of a desktop computer. Everything is in a separate Virtual Machine. Your spreadsheet and your browser might as well be on two AWS instances. And, yes, USB widgets are problematic enough that they run in their own VM.
Yes, I’ve casually read a bit about Qubes, but their discussion of USB security was in no way reassuring. Computers are to malice what a sieve is to rainfall. No good at all.
Yeah, when they have to set up their own USB quarantine area, that doesn’t say much about the underlying security.
Have you heard about “evil maid” attacks and rubber duckies?
Indeed
Total xfer — about 44G. System — 8G Raspberry Pi / Ubuntu MATE. Input — USB3 SABRENT Disk dock. Output — USB3 WD “My Book” 6T drive. Peaked at 20M/sec….don’t even ask about low spots.
I originally built this rig for salvaging data off of all the pulled drives that were in computers accessible to the 2015 malware. Uses ARM instead of Intel SW, mounts with Linux protections (RO). It’s been behind the plumbing/car troubles/refrigerator problems/relationship issues/other fire drills for some months, but when Fiancee had her problem it was ready for action.
I got the disk dock off of Woot because it was a screamin’ deal….*sigh*…..like the old Woot. Now that I know how well it works, I wish I’d gotten three.
Yesterday’s comments section was closing in on 700 when I last looked. And the page was taking about 2.5 seconds to respond to actions like hovering over an unread comment and waiting to see it clear the yellow background.
At the last, there (after 23:00), the comment #’s in the green orb would stall and I’d have to reload and go back and find the stragglers.
Oooh…and I managed a double-post for the first time in ages!!!
I’m actually starting to get weird stuff now – which I did NOT when the site was in paging mode.
If I may vote, the pagination was a pain, but worked better, IMO.
I think I will take a vote later. I think I’d like to watch the new setting fail another day!
One possibility is to increase the “more” setting, too, because I think that figures in, but I’m not sure that will solve all the problems. I am seeing weirdness earlier than the first pagination / load more. with the “load more” model.
Showing my ignorance, again.
#1 Yellow shaded posts to easily identify posts that I have not read is HUGE.
The orb color, count and all of that is not significant in any functional way. IMO.
The bell notifier indicating a post to mine is helpful to further a personal discussion point.
That’s about where I come from. The orange simply tells me it’s worth while scrolling to look for yellow.
I hate the bell notifier for a couple reasons. I often don’t know where in the thread the comment is coming from. If I like a comment from the bell notifier it disappears if I back out of the notifier.
I really like the yellow highlights and the orange ball.
Thanks! Just so you know this, then, some useful info. The bell notifier is part of a tool called JetPack that helps WordPress.com registered members primarily, because JetPack is a WordPress.com product.
The orb, yellow shading, and most of the “chrome” on our commenting system is a plugin – wpDiscuz. That plugin reacts to different WordPress (vanilla WordPress) settings, including pagination of comments.
I haven’t had ANY problems whatsoever.
For this guest, it’s been working perfectly, since stroke of midnight yesterday (34 hours straight).
Is the “guest” the baseline, or is the “registered” the baseline?
Choices, choices …
Wolfie, I load “First Page’ early in the day AND NEVER REFRESH.
I place cursor on the yellow new comments to turn them white.
In this way I do not get ‘Pagnation’ unless the loading is way too slow and then I just refresh and I get stuff separated into pages (Usually three or more)
That gives you the best of both worlds although I do have to page through the comments to see the new ones.
I spotted you typed ‘pagnation’ and figured it was a typo.
Then I realized you might have meant “pagination-stagnation” and thought maybe it wasn’t.
Yeah…
Something like Raider Pagnation 
One less bell to answer? 😆
(sorry) (great tune, great vid I just heard):
I agree with DP. Pagination worked better.
My experience was perfect until the amount of comments made everything super sluggish.
Is there any way to make pagination happen at around 4 or 5 hundred comments, instead of 250?
Yes, I’m still bothered by the fact that it WAS perfect for a while. But the behavior in Steve’s post immediately seemed to have been messed up by the bad behavior in other tabs with yesterday’s open – or at least that’s the way it LOOKED.
For instance, I just did a reload with 644 in the green orb…..and it’s now 646.
I think it’s clear that paging mode and “load more” mode are BOTH quirky.
And you can’t even see the quirks until they’re under load.
But I’m seeing the quirks right now, with almost no load!
Did you see my solution to your “I want to know the time of my last refresh” puzzle? Posted to you in yesterday’s comments in response to “essentially”.
I saw that, but have been too scattered to try it. It looks good and I hope it works, but today has been like trying to read under strobe lights.
Nicely written piece Steve.
I appreciate Ernest Rutherford far more now.
Turns out, he did a bunch of stuff I had vaguely figured that Bohr and Fermi did.
So, which one was the time traveler from the future? Rutherford, Tesla, or Einstein?
Wolf’s AND logic comes to mind.
Trick question! Rutherford was from the future, Tesla was from an alternate future, and Einstein was from an alien future….
I was genuinely surprised at how much he did. We (geeky types) all have heard about the gold foil experiment, yet he did a LOT of basic work in radioactivity long before that.
I think as I continue on we’ll find that Bohr and Fermi did indeed a LOT but it was standing on his shoulders.
Rutherford was in very early, almost as early as the Curies. The Curies get a lot of popular attention (maybe the wife/husband aspect of it has something to do with that); but even in the specific topic they were working at Rutherford seems to have done almost as much (he was working with thorium, they were working with uranium). Rutherford was literally there when the electron was discovered; Wiki seems to think his doing things with X rays was a contributor to that discovery.
Agreed! Very enjoyable, and a rather wild ride through radioactivity!
New Schlichter —
https://townhall.com/columnists/kurtschlichter/2021/08/05/liberals-hate-that-you-have-rights-n2593564
No, liberals hate that anyone who disagrees with them have rights…
…much less are allowed to live and breathe.
But they are working hard on their problem.
“Too cute” – from Gab! (for extra irony)
Sadly, the people who need to hear this won’t understand it.
Most hilarious fact-check I’ve ever read! Referring to the author of a hit job on Mike Lindell:
Love that TGP is now FACT-CHECKING THE MSM!!!
See:
https://tgpfactcheck.com/shameful-atlantics-dishonest-a-baum-applebaums-hit-piece-on-mike-lindell/
Another excellent lecture, very well presented.
And just the right kind of CRT (cathode ray tube)!
I’m off to do some practical work in the footsteps of Hertz and Marconi.
In the video interview of the J6 prisoner posted yesterday, I thought his statement about why the excessive prosecution might have happened was worth thinking about.
He said, lots of people witnessed police abuse (of peaceful Trump supporters). So [the Fibbies] go overboard intimidating and making an example of people that were there so that people would not step forward and testify about what they saw.
Not saying that’s the whole story, but it is an important point.
CPAs know this about tax prosecutions. The IRS will RAM people hard who might have witnessed previous IRS misbehavior. Goes with the unaccountable bureaucrat territory.
The Cops even caused a woman to suffocate to death.
Philip Anderson: Capitol Police Killed Rosanne Boyland on Jan. 6 – “She Was Holding My Hand When She Died”
and another witness
NEW VIDEO: Rosanne Boyland Killed by Cops – Police Prevent Her Rescue – Push Protesters Down Steps – Beat Rosanne and Protesters with Sticks — Protesters Attempt CPR
and
THE POLICE KILLED HER: Three More Eye-Witnesses Who Were Later Arrested Speak Out on Police Killing of Jan. 6 Protester Rosanne Boyland – Here Are Their Stories
How about some simple math for perspective?
.
.
Current world population = 7,781,000,000
Current US population = 332,000,000
https://www.census.gov/popclock/
Total # of people who have contracted WuFlu worldwide = 202,000,000
Total worldwide deaths (claimed) = 4,000,000
Total # cases USA = 36,000,000
Total deaths USA = 632,000
https://www.worldometers.info/coronavirus/
.
.
So…….
Worldwide:
Only .0256, or 2.5%, of the entire world’s population has gotten WuFlu to date.
Only .00051, or .051%, of the entire world’s population has died from it. THIS IS 1/2 of 1/2 OF A PERCENT.
.
In the USA:
10.8% of the US pop. have gotten the WuFlu
.0019, or .19%, of the US pop. has died from it.
Got that?
.
Now…..
The US makes up just 4.2% of the total world population…
….BUT….
According to THEIR figures, the US is responsible for 17.8% of the total number of WuFlu cases worldwide…
….and 15.8% of all deaths resulting from it.
😏
.
We need a comparison nation, just for giggles. How about China?
China pop. = 1,445,000,000
Total # cases = 93,000
Total deaths = 4,600
% of world pop. = 18.2% (largest pop. in the world)
% of world cases = .046%
% of world deaths = .0022%
.
.
Either the numbers are wrong – or – the US was targeted and the pathogen intentionally spread to a population that was possibly intentionally made vulnerable by flu vaccines.
No matter for whom it was targeted, it’s been a living HELL over here. And with the Greens, Die Linke, the SPD, the FDP, and the now-leftist CDU/CSU (thanks, not, Merde-Kuh) pushing this and the Gore-Bull warming cr@p, it’s not going to get better anytime soon. And we’ve got elections in September, and it looks like the sheeple will be conned into voting for the Greens (Annalena Baerbock, WEF “young leader”, plagarist, liar, and globalist puppet handmaiden of Merde-Kuh)…..
We need a HUGE amount of prayer… A covering prayer…
(btw howdy, good to see you here!)…
This really shows the fraud. The fraud was biggest in the US. Because we had a political party invested in it.
Plus, you were generous by saying 1/2 of 1/2 of a percent – 0.051 is 1/2 of 1/10 of a percent!!! Even smaller. It’s ridiculously small.
Thank you. Yes.
So small, in fact, that the FEAR and ALARM being pumped and dumped is ridiculously overblown, esp. with the benefit of hindsight.
They want us to give us our freedoms today, forever, for the PROMISE (with no guarantee…or accountability) of “safety”.
It is a fool’s bargain.
The Bible reference to “peace and safety” ..
1 Thessalonians 5:3
Verse Concepts
While they are saying, “Peace and safety!” then destruction will come upon them suddenly like labor pains upon a woman with child, and they will not escape.
1 Thessalonians 5:1-3
Now as to the times and the epochs, brethren, you have no need of anything to be written to you. For you yourselves know full well that the day of the Lord will come just like a thief in the night. While they are saying, “Peace and safety!” then destruction will come upon them suddenly like labor pains upon a woman with child, and they will not escape.
Source: https://bible.knowing-jesus.com/topics/Peace-And-Safety
Hold fast … and God bless
We were also more aggressive with testing. I imagine a lot of people in the poorer parts of the world caught it and dealt with it without ever seeing a doctor.
If the testing were valid, at least, that would make sense as a possible explanation. Even if the testing were invalid, it was probably run on a greater percentage of our population than elsewhere, so whatever it was it DID read out on (condition X, mislabeled as Covid) would be detected in more of our people than elsewhere.
Keep ASKING the right questions.
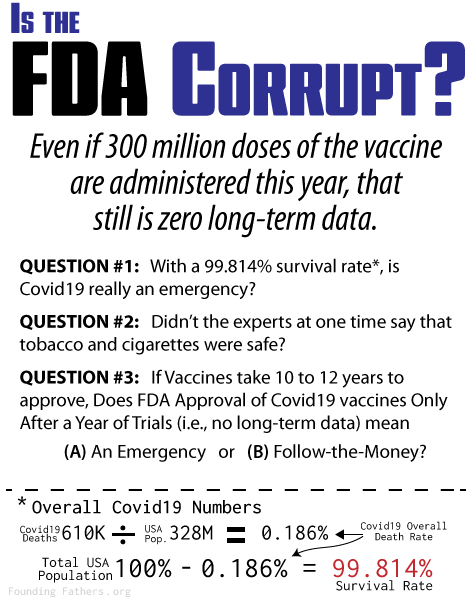
Filing this one away as interesting.
Vox Cantoris is usually spot on.
I’ve seen reports that Francis is long gone, and that’s an actor. No idea if it is true or not, but this story leaking could be a cover story for the eventual exit.
Well, that’s a bit too far for me to go. I think he’s around, but not for long.
I’m going with AND logic. He uses the actor to greet grabby Chinese audience members now! 😉
I’m filing the cancer news away as one of the best and most hoped for rumors out of that snake pit in many, many years.
I said about the same thing the minute his order came down to destroy the Mass of the ages.
To say the clergy have been disappointing in this whole thing is an understatement.
Catholic Church Receives Billions from the USA, Illegal Immigration Proves Very Profitable — collects $1.6 billion in U.S. contracts, grants since 2012
others are just as bad.
U.S. Lutherans Make Entire Denomination an Illegal Alien “Sanctuary”
North American Baptists called to embrace immigrants and refugees
Maurice Strong, puppet of David Rockefeller must of loved this last guy.
Parliment of the World’s Religions — Climate Action 2021
Yeah, Maurice Strong and the Cabal must really love this guy.
NOTICE HOW JOHNSON KEPT CHURCHES FROM ‘interfering’ in COMMIE politics
http://hushmoney.org/501c3-facts.htm
but this bupkis is A-OK!
I have a lot to say on the subject. Most of the money actually goes to Catholic Charities which is not technically part of the Church, but a separate non-profit. Same with a good number of schools, especially if they are run by an order.
Then there was the 1832 deal which I am hoping goes away when the Rothschilds are no longer part of our lives.
That’s how the Church hides the income…having technically non-profit and non-Church charities with separate budgets, etc.
It’s all a scam. Don’t ask me how I know.
Sellers…. wonder if he’s related to that twerp in Arizona??????
In any case, Rob Sellers is a real piece of work. And not good work, at that.
Somehow the term “Crockodile Christian” came to mind. He claims to be a Christian, but just eats away at your soul until nothing is left; i.e. a sly, conniving heretic…
Thoughts? This does not look like lightning.
https://twitter.com/Akshay80307230/status/1423724361895354372
That is EXACTLY lightning. You can even see it!
Yep. Now that’s the real Rod from God. The lightning rod. 😄
AMEN!!! 😉
From Mt. Olympus. It’s a known fact Jupiter does not like upstarts.
Thank you for the history lesson, Steve. I love thinking about these scientific discoveries that way.
Also, I like how an atom looks like a solar system.
Signed,
The English Major 😄
Wellllll….it’s going to turn out later to look more like bizarro world. But it is a useful analogy nevertheless.
😄
Verse of the Day for Saturday, August 7, 2021
✟
“For in him we live, and move, and have our being…”
Acts 17:28 (KJV)
Thank You, Jesus, for blessings received and prayers answered!!!
BE MY VOICE
PRAYING ON THE ARMOR OF GOD
Father God, I now follow your command to put on the full armor of God, because my battle is not against flesh and blood but against rulers, authorities, the powers of this dark world and against spiritual forces of evil in the unseen world.
I first pray on the Belt of Truth that it may be buckled around my waist, may I be centered and encircled by your truth dear Lord. Hem me inside all that is true and right, and may I be protected and held up by the truth of your living word, in my Lord Jesus name.
I pray on the Breastplate of righteousness, please protect my vital organs and my inner man, cover my integrity, my spirit, and my soul. Guard my heart for it is the wellspring of life, please strengthen and guard the most vulnerable places in my life with that which is right, good, and noble that I might not receive a fatal blow from the enemy, in my Lord Jesus name.
I pray on the Gospel Shoes of Peace. I choose to stand in the shoes of your good news, and on the firm foundation of my Lord and Savior Jesus Christ, the solid eternal rock. All other ground is sinking sand, I pray that I will not slip or fall, but that my feet would be firmly fitted on your lordship, my Lord Jesus. I choose to stand on you, so that the peace of God, which transcends all understanding will guard my heart and mind in Christ Jesus, the eternal Rock of Ages. I receive your holy peace now my Lord, from the sole of my feet to the crown of my head, in my Lord Jesus name.
I pray the Shield of Faith into my hand now. As I take up the shield of faith, I ask that you might extinguish every dart and arrow, that is launched from the enemy to take me down spiritually, physically, mentally, emotionally, and every attempt of the enemy to destroy my joy. I ask that my faith in you would make it flame out. Extinguish every flaming arrow that would come against me, my life, my family, my home, or my ministry. May my faith always be out in front of me like a shield. Give me the courage to “faith my fears” by choosing to walk by faith and not by sight, in my Lord Jesus name.
I pray on the Helmet of Salvation, that you might protect my mind from the thoughts that can lead me astray. I choose to take every thought captive, and arrest all intentioned ideas and motives that would harm others, or distract me from your holy will for me. I submit every captured thought to the Lordship of my Lord Jesus Christ, and ask that you would imprison those thoughts that are not of you my Lord. Transform my mind and renew my thinking that I may think God thoughts, and have a sober mind that is focused on your glory. Please protect me from being double minded that I may allow my mind, I reject to live an earthly life, because I choose to live a holy one, governed by you My Lord Jesus, the prince of peace, please have my mind to be saturated with the holy mind of Christ, in my Lord Jesus name.
Finally, I take up the Sword of the Spirit which is the holy word of God, I pray this powerful offensive weapon into my hand, and ask that your holy word would be fitting for every encounter I face. As the enemy gets close to me, please give me the insight, wisdom, and skill to wield the word of God to drive away the enemy, in my Lord Jesus name.
May the enemy and his team flee from me, upon hearing the word of God spoken by the power and direction of the Holy Spirit. Give me the sword of the spirit to cut through the wiles of the devil, so that I may discern the schemes of the enemy when he is near.
With all kinds of prayers, supplication, and intercession I pray to you my Lord God as the one who fights my battles. Now that I’m in your holy powerful armor, I walk away covered and ready to face my day as you go before me, and please protect me in the midst of the spiritual warfare in this unseen world, in my Lord Jesus name.
Thank you my Lord, for the spiritual weapons of armor and prayer that you have given me. It is written no weapon formed against me shall prosper, and you will refute every tongue that accuses me.
Thank you Father God, my Lord Jesus and the Holy Spirit, that I am more than a conqueror in my Lord Jesus. I pray all of this in the mighty name of my Lord God and Savior Jesus Christ, amen.
Even I understand science enough to know that without a “control group” scientific conclusions about anything are virtually impossible. I put this in a reply to Wolf on another post this morning, but I want to be sure we all see it. It’s from CTH:
This is Nuts, Moderna and Pfizer Intentionally Lost The Clinical Trial Control Group Testing Vaccine Efficacy and Safety
Here is the imbedded article at NPR:
https://www.npr.org/sections/health-shots/2021/02/19/969143015/long-term-studies-of-covid-19-vaccines-hurt-by-placebo-recipients-getting-immuni
This is nucking futs!
You can’t just make up some shit as a vaccine and inject it into people without randomized trials with control groups. We might as well be in the Middle Ages, when “physicians” rolled ox dung into pills for rheumatism, or used hanged-mans grease (rendered fat from dead criminals) on achy joints. Yes, they did that:
https://www.historyonthenet.com/crazy-potions-and-nasty-nostrums-six-bizarre-medieval-medicines
And people think I’M nuts because I make medicine out of backyard plants!
We are in faux-science driven hell at this point. It’s just unbelievable.
I never dreamed that Trump would be able to unmask “Fake Science”, but here we are!!!
YUPPERS!!!
I LOVE watching them crash and burn.
It’s really just one crazy revelation after another nowadays.
Hmmm…
Looks like @Johnheretohelp and @LuedersLyndon Twitter accounts are suspended.
Musta been “over the target” !
😉
Quick update :
(Guess who’s on Gab ?!! 😉 ) :
@Johnheretohelp
49m
·
Good morning everyone thank you so much for your support and for following me over here. The Twitter saga is not over by any means.
I would like to document today Saturday August 7th 2021. That last night I was threatened by a federal agent that I have had zero contact with in years. This is one of the people who tortured me in 2009/10.
Apparently he saw information posted online about the dirty trick Squad, and it did not have my name anywhere on it. But he knew immediately where it came from. He did not question the information or claim that it was wrong in any way, he can’t and he knows he’s neck deep in it, instead he moved immediately to threats.
So how was your morning? https://gab.com/emoji/1f923.svghttps://gab.com/emoji/1f923.svghttps://gab.com/emoji/1f437.svghttps://gab.com/emoji/1f437.svg
I’ll be perfectly honest, I don’t believe people any more when they claim some federal agent threatened them.
It’s too easy to imagine a bullshitter making false claims like that to try to lend themselves credibility.
My experience with .gov types is typically of the following natures:
Typically the opposite of threats. Of course, if one talks about any of this stuff, one risks being classified as a bullshitter, just as much as if one says “they threatened me”. One simply has to buck up and take the hits. Eventually, one can fight back in the same style.
One of the few things I do believe JHTH about without too much question is that Radium Rod was part of some “dirty tricks squad”. It’s the best explanation for several nasty incidents where I came close to being “January Sixthed”.
Now it’s entirely possible that JHTH is being used to discredit the idea of Rosenstein being a bad guy – that he’s an “immunization”. I can believe THAT, too.
But based on the above list, I think our wonderful feds do set people up.
Especially people like us.
JHTH was under oath when that “interview” happened. It was actually a deposition according to Lin Wood who was the interviewer.
Had the black bar this morning, left at 6:30am CDT, came back at 9 and it was gone.
I had been logged out. I logged back in and bar is back. One oddity, I was looking at the Thumbs up numbers, because they turn green when I click them, and although some were grey, I was listed in the list with the number next to the thumb. After logging back in my name and icon had turned into Guest.
We are back 🙂
Family room at least picked up and swept and dusted. Pre-teen boys…yeah.
What I was trying to post when the site went down.
https://twitter.com/ScorpioJanie/status/1423756036813512706
She is good and spot on 🙂
At what point does the compliant sheep person start to tell the gov’t to STFU and call it stupid?
Ask for my friend Patience.
The sheep person’s judgement is so clouded by fear that there isn’t anything stupid about it even if it’s really asinine to those who are not afraid.
BUT THE VIRAL LOAD IS HIGHER IN THE VACCINATED! THE VACCINES MAKE THE PROBLEM WORSE! ROCHELLE “THE LIAR” WALENSKY IS TRYING TO CONVINCE PEOPLE THAT THE LOAD IS THE SAME, TO HIDE ADE!!!
This is a major, major point.
Massive.
All caps is appropriate.
This is where the point was first evident:
It appears to me that the CDC and the UK Health Ministry, and probably others, are trying to minimize ADE to “not effective for the new variant” rather than “worse than useless on the increasingly virulent virus”. That allows them to keep pushing the vaccines that keep pushing virulence of the virus (or provide cover for release of increasingly virulent variants).
This is actually EXTREMELY dangerous and EXTREMELY underhanded and sinister.
The only way for humanity to win this game is NOT TO PLAY.
Wolf Moon
Yours truly tried telling this to my SISTER today — she’s “fully vaccinated”, but now has COVID, plus STREP THROAT. She’s convinced that she got COVID from “the unvaccinated.” She’s going to get the BOOSTER SHOT. She flat-out said that she’s “following the science.” Dismissed all that I told her.
Sorry to hear this! These people are following Democrats, not science. It’s sad, but they’re trusting criminals.
I went to ScorpioJanie on Twitter and scrolled down a little. Took a screenshot that shows a still-visible post “is no longer available because it violates the Twitter rules.” I am amused that they didn’t actually delete the content on the feed. 
Holy crap.
We should watch for things like this that might indicate they are getting sloppy.
Test.
Are the tiny peni DS scumbags doing another DDoS attack here again?
Something sure happened but it seems we are back.
However, you’re assuming gender; they may be cumdumpster female agents.
SteveInCO
Thanks so much for another great opener today. Stuff like using “kablooey” and “lying sack of bearded dragon shit” kept me reading, for sure — and I learned things.
Hmmm, “cumdumpster female agents” — like, maybe, possibly, a “certain person” who never showed up to our host’s “poker game put up or shut up” challenge?
Someone must be afraid of your science, Steve. 😎
That’s an interesting point – they shouldn’t be.
What time was the site down? I was away for a while.
Around noon today for me, just prior to my post above.
Right now, @ 5, all seems well.
Based on posts on this page…
Last post before down was SingingSoul at 10:51
First post after back up was DP at 12:45
New type of failure since it was cPanel serving the 404
Also, I was able to use browser back button to get to yesterdays page while this page was down FOR a WINDOW of time.
Felt like the elves were moving furniture behind the curtain.
Quick ground report, but 1st access issues. I was reading yesterday’s thread yesterday & today & things were Really Slow, but figured it was my system (screaming from too many windows/tabs, per usual). After restart all Q-Tree was at 404. Signed into WP & got the ribbon & could see replies to me on Q-Tree posts but my replies to them didn’t go through. Went to U-Tree & was reading to see if others were having issues, but didn’t finish that read before clicking on someone else’s Q-Tree link & getting back in here…
Husband’s rock cover band had concerts last Sunday & last night. Sunday was in a West-side metro-Detroit more blue-collar area. Masks were present on some people, probably less than 20%. Last night was an East-side, likely more affluent suburb. There were maybe masks on 2% of patrons. Both crowds seemed to not be concerned w/ “social distancing”. It didn’t look like people who would relish returns to mask nazi-ism.
We’re about to leave for a concert w/ his other band, Christian blues/rock/originals/some praise & that venue is a couple hours away, near the state’s capital. The hosting band is black (the other venues were mostly white) so I’ll try to note & pass on any covidiocy observations.
Oh, last Sunday I missed a discussion w/ the band guys & someone at the venue that my husband described as “my people” as they were expounding on the BS going on in the country & informed on at least some of what we discuss around here. All of those guys are more salt-of-the-earth/blue collar types.
Last night I spoke to one of the band mates, Michael had said he is reading Levin’s American Marxism, & he indicated he & some buddies were thinking of starting a v-log, patriotic style stuff, but are concerned about exposure & risks to their families. I’m going to share some of our sources w/ him at a later time & see if he might be interested in broadening his resource base, maybe he’ll even stop by here if we’re lucky.
Well, gotta run. God Bless you all. It’s busy around here between music & wedding prep for my daughter’s upcoming wedding. Thanks for any thoughts or prayers!
Scratching my head over this one:
Mike Lindell Drops Bombshell: ABC, NBC, CBS Are All Running The Ad FOX News Rejected
https://www.thegatewaypundit.com/2021/08/mike-lindell-drops-bombshell-abc-nbc-cbs-running-ad-fox-news-rejected/
FOX = Rupert Murddock NINETY YEARS OF AGE…
This is a LONG article I have posted a lot of snippets.
NYT — Murdoch’s Dealings in China: It’s Business, and It’s Personal
Murdoch IS OWNED BY THE CHINESE…
OWNED is right!!
WE aren’t surprised but I sure hope some of the snowflakes see it…
Wut? No vaxx mandate for hussein family party?
It was justa few weeks ago BIG MIKE made a big fuss how him and his family would not mingle with anyone NOT injected.
Hypocrites.
https://theconservativetreehouse.com/blog/2021/08/07/pathologist-dr-ryan-cole-delivers-concerning-message-about-covid-vaccine-and-long-term-impacts/?utm_source=rss&utm_medium=rss&utm_campaign=pathologist-dr-ryan-cole-delivers-concerning-message-about-covid-vaccine-and-long-term-impacts
grandmaintexas
Whoa.
Dr. Cole, the MD in the linked video, issued a statement after he got criticized for his views.
He’s aghast that what he assumed was a presentation to a “small group of people” became viral.
The below linked article about Dr. Cole’s statement also includes his admission that he himself AND HIS FAMILY are all “vaccinated.”
How can Dr. Cole, who describes getting “vaccinated” is akin to crossing the Rubicon, that it’s something that cannot be reversed, that the spike protein in the “vaccines” will continue to replicate inside the body — How can Dr. Cole, who obviously has studied the dangers and flaws of the “vaccines”, ALSO go ahead and do the “follow the science” thing and get himself and his family injected with the same “vaccines”??
http://www.ktvb.com/article/news/local/208/garden-city-doctor-reacts-fact-checking-his-statements-lawmakers/
Wow!!! Wth??? Insanity! He had to know it was going to be filmed and shared.
That link is broken.
Hahaha What mind bending message can Garden Gnome and his sidekick Walesky come up with to explain this ???? ( my question..why are they being tested if asymptomatic , for fun, addicted ??)
https://nypost.com/2021/06/10/passengers-test-positive-for-covid-on-fully-vaxxed-ship/
The two passengers are asymptomatic but in isolation while the cruise line conducts contact tracing, the statement said.
All passengers and crew have to show proof that they’re fully vaccinated, reports said.
“All guests on Celebrity Millennium were required to show proof of vaccination as well as a negative COVID-19 test within 72 hours before sailing from St. Maarten this past Saturday,” the statement said.
Makes it really convenient to stop anyone, anytime.
This seems like they are pivoting away from the unvaxxed narrative to a narrative that allows them to stop and detain people because possible ‘positive covid test.’
On-the-spot testing next? New tests coming out from the hellish Gates guy? Would you trust the cleanliness of those new tests?
Can’t go in the restaurant unless you take an instant covid test…
Could be. The new axis of evil with Gates and Soros will NOT pass without a fresh round of CV mayhem….
Yeah, about those tests, yesterday the all day family deal that I was forcing everyone to read , lol……I had no idea that there already is OTC tests, instant results but I’m sure these aren’t “official” but official enough for the moron pediatrician to run like a scared rabbit
NOT taking a Covid test simply because some liar in government or a business says I should.
I’m wondering if the key is contact tracing. Someone “positive” came in contact with them, perhaps?
Admittedly, I am pretty slow.
For twenty months of this Covid cluster f@ck, I have viewed “contact tracing” as 100% folly. Just more government and health care car follies.
Agree about contact tracing. It’s another way to ensnare people who are just going about their lives.
Today in American Revolutionary War History:
August 7, 1782 – In Newburgh, NY, General George Washington created the “Badge of Military Merit.” On Washington’s 200th birthday (February 22, 1932) the U.S. War Department announced the creation of the “Order of the Purple Heart.”
https://on-this-day.com/cgi-bin/otd/amrevotd.pl#
“It’s not tyranny we desire; it’s a just, limited, federal government.”
Alexander Hamilton
Apple and security?
The Problem with Perceptual Hashes (rentafounder.com)
Good article.
Apple is going woke/broke in so many ways.
Acting like idiots.
I am dumping ALL my Apple stuff. This makes life much easier.
There is no way to trust them now. They went too far down this road. They’re filled with wokies. Taken over.
I worry about hash collisions. Not supposed to happen, but I’ve seen it in the database world a number of times… Folks in the HP3000 MPE world might know that as “migrating secondaries”…
It’s a PRETEXT. The whole thing is a freakin’ pretext for surveillance. It will flip to “insurrectionists” in a HEARTBEAT.
Yes….
The same goals to be accomplished with smart meters on water meters, and the new electric meters that were installed with satellite connectivity. The gas meters were moved out of all the houses without owners having any say in where they went resulting in several attacked to the front. Sooner or later they will have to be replaced as well weather being what it is.
Control ratcheted up piece by piece.
And by looking at the “smart” electrical meter’s usage profile(s), it’s possible to tell which appliances are being used, and in some cases, which channel is being watched on the TV. A couple of years ago a study on this was done in the UK, who are all in on the “smart meter” craze (thanks to Millibrain &co.) and the results were, erm, shocking. And the folks in the UK appear to be part of a testing ground for the globalists and their invasive, controlling policies…
Smart meters from stupid, power-mad “governments”….
I can see it now: make a faux pas on (anti-)social media, and poof! water, gas, electricity, internet, telephone and anything else they can figure out gets shut off…..
Seth Keshel
Tale 1: You go to the doctor for a checkup. A lab tech takes a blood sample and a few days later, the doctor calls you up and says that while your general health outlook appears stable, you have a few lab values that are off that he would like to evaluate, as they may align with symptoms of serious disease. You’re busy and shrug it off, and four years later, die of something that was treatable if attacked early.
Tale 2: You live in a GOP state and have a presidential election. Data experts evaluate the certified results, and some time later they call up the politicians and consultants and say that while the general health appears stable (the state remained Republican) you have a handful of counties that appear to be off that you’d like to evaluate, as they may align with symptoms of serious election fraud. You’re busy searching for donations and allowing this under the table, and four years later, your state is taken over by people it has no intention of electing at all.
– – –
Sound familiar? This is the argument for audits. These are OUR ballots and OUR processes, not theirs. This is not just about data. Affidavits are sworn under penalty of perjury and are thus considered evidence.
In Texas, we have countless affidavits alleging drive-through voting fraud, with perps coming through with a handful of drivers licenses and receiving as many ballots to return themselves right in the lane. Ask Bianca Garcia about that.
We have a Democrat operative spilling the beans on the election fraud ring in our country’s third largest city, Houston. We have obvious counties in the reddest parts of Texas that appear to have electronic fraud. Our suburban counties have record GOP growth % on top of an already high total of votes, only to be surpassed by 60-80% democrat vote gains in the same counties in the same year.
And this is just Texas. The politicians cannot deny the people our transparency. They work for us. They need to get that straight. If your state doesn’t think they have an issue, press them to give an opinion on the absolute gutter trash “election” conducted in PA, MI, WI, MN, AZ, NV, GA and ask if they think that disenfranchised the voters and electors of their own state. That was the spirit of Texas vs. Pennsylvania.
History will not remember the names of the cowards who refused to guarantee election integrity. I’m glad they think they’re right – now it is time to prove it. If you prove us wrong, it means you proved we have sustainable elections and thus are doing good things for this country. We will acknowledge that. But you won’t attempt to prove us wrong.
We will persist.
CodeMonkeyZ
Work on phase 1 continues.
Will have more information out about it after Mike Lindell’s Cyber Symposium.
CodeMonkeyZ
Ambush.
Counter-ambush.
A hideous plot uncovered.
We explored this at least once before about a month or so back but not in too much detail.
While we correctly espouse the hold the line with no vax – no way, some of us are going to come across friends or family members that feel the same way but for whatever reason feel they need their job or need to travel for family reasons. Besides medical and religious exemptions what other advise do you tell someone that feels they have no choice but to take the jab?
What I’m asking about is how do you beat the jab by making it less effective?
Blood thinners before and after the jab? Has seen advise saying don’t take before the jab as the vax will be less effective.
Vitamin C in the highest tolerable does? Keep things slippery on the inside so vax can pass through easier? So work yourself up to high dosages before the jab.
Diuretics and additional water consumption? To help flush the system.
What else or are some of those bad or useless ideas?
Some people close to me are vaccinated. Every day there are new reasons to be concerned about them, and I wonder if things can be done after vaccination to mitigate the effects.
See my response to para.
Thank you.
First choice: hold the line.
Second choice: cause as much damage to the bullies (make them fire you, get a lawyer).
Last choice: take a chance on death or lifetime of failing immune function.
For those that make the last choice… (wrong choice)
Look for things the FDA clamped down on.
Ask the question, “what are they most afraid of?”
Look for things that prevent virus from getting into cells (like ivermectin?)
Look for things that break down graphene (just in case that’s in play).
Make sure your lymphatic and organs of elimination are in great shape.
Take a long as possible to delay the kill shot.
Go to confession.
Tell your family you love them more than anything.
Update your will.
Ask the God that turned the water into wine,
to turn the vaxx into water.
One posits that now is the time to secure HCQ from whatever source is possible.
Ditto with Ivermectin — betcha the time is coming that getting animal Ivermectin anywhere will require an Rx from either a veterinarian (and also will require proof that the person requesting the Rx has a dog, cat, horse, or other animal); or that human-use Ivermectin will require an Rx from an in-person visit with an MD.
People will find a way. As an example, you could go to a farmer and purchase a cow, get ivermectin “for your cow”, and fill a couple of freezers a couple of weeks later.
People with cows are going to have lots of new friends.
Black market.
Good in put. Wonders if we need to break it down to
Pre shot prep may mean stop taking certain vitamins that make cells more receptive.
Like should you stop Zinc or Selenium before taking the vax and if so for how long… before and after?
This topic may need to come up again, possibly as separate post. Guberment is still pushing and lots of folk are going to need repair if possible at all.
Ok. I’ll give you the best I’ve got for vaccine injury avoidance and treatment herbally.
1) Star anise tea. Strengthens the blood vessels.
Make a tea by grinding the whole pod and steeping in boiling hot water in a covered cup for 1/2 an hour or longer.
2) Yellowdock Root. Flushes the lymphatic system.
3) Dandelion Root. Diuretic, flushes the pancreas and liver.
4) Burdock Root. Blood tonic, lymphatic tonic.
I mix these last three together in a tea. Simmer a tablespoon of the blend in a cup of water for 10 minutes, in a covered pan. Cool, strain, and drink.
5) Lady’s Mantle. For excessive menstruation and/or bleeding between periods. Uterine astringent.
Make into a tea.
6) Bromelain. Inhibits blood platelet aggregation.
This is often found combined with quercetin in capsule form. It also helps to quercetin to absorb into the system.
As always, anyone can have an allergic reaction to anything at anytime. So when you are trying something new, don’t be by yourself to do it.
These are good. Wonders if we need to break it down to
Pre shot prep may mean stop taking certain vitamins that make cells more receptive.
Like should you stop Zinc or Selenium before taking the vax and if so for how long… before and after?
This topic may need to come up again, possibly as separate post. Guberment is still pushing and lots of folk are going to need repair if possible at all.
Yes. This is a complex topic. I may have to do an entire post about the herbal options if Wolf would let me.
I don’t know how much they will help, but I know what they do. The exact opposite of the stupid shot. I have no idea what it does, and it does NOT help.
A post on this would be great!
I don’t think there is any “letting” involved. Do it!
Ok. It might take me some time. I’m going camping for a few days, no internet access. Which will be good for me.
YES!
Bromelain. Just was reading today about the use of proteolytic enzymes (enzymes that help break down proteins). I’ve used them for a very long time to break down excess mucus in the sinuses. (Allergy problems.)
Makes sense now that I think about it.
We are wanting to help the body break down foreign proteins.
Spike proteins to be specific.
Food sources: Pineapple, Ginger, Papaya, Kiwi, Sauerkraut, Yogurt, Kefir, Miso
Supplement form: Nattokinase, Serrapeptase (eg. Repair Gold)
Dr. Axe article: https://draxe.com/nutrition/proteolytic-enzymes/
I like food sources whenever possible. Thanks! That is good info, and from personal experience.
I just made a batch of homemade sauerkraut, too.
Looking forward to your post.
A little Rural Life for ya.

https://gab.com/AutumnFields/posts/106717559710191626
Beautiful!
I take Now Foods Quercetin w/Bromelian daily. 👍
That’s good! I just ordered that one a few days ago.
Q
@QAnon211
1h
In #ITALY today!! People are BURNING THEIR JAB PASSPORTS!!! PROTESTS HAVE ERRUPTED IN 12 CITIES AS PEOPLE CHANT “BORN FREE & WE’LL DIE FREE”
[video src="https://media.gab.com/system/media_attachments/files/081/352/652/original/f3df4f7da215e788.mp4" /]
I’m in awe of these brave souls. Brava
Italy would have a MIGA government except for FAKE ELECTIONS.
Went to the Walmart today.
Audio blaring about the covid shot…available at the pharmacy!
Masked greeter back at the door, probably anticipating the masking syndrome to gear up again.
Same at the grocery stores here.
And, there are now television ads for fancy masks that can just hang from the neck and be put on when needed.
They aren’t kidding around. They intend to go full on again.
yes, they do.
I don’t know that it’s going to work for anyone other than the true believers.
I went grocery shopping yesterday. Employees are back to wearing masks at all of the stores. Noticeable uptick in masked customers, but the unmasked are still the majority by far.
One of the most dystopic moments I experienced from this whole psy-op was a few months ago was when I was grocery shopping. Out of nowhere on the loudspeaker: “The vaccine is here. Everything will be better now.” Creepy.
😖
All employees everywhere here have had to wear them. No one has stopped. Medical has had to continue. Now you have to take covid tests starting next month to go into hospitals or snfs.
Guess I’ll go back to skipping appointments as I did most of 2020.
Dunno if they’ll do prescription by TELEMED or whatever that latest gimmick is.
NOT taking a Covid test unless I have multiple symptoms.
Not taking a Covid test, period. I might’ve considered it back in the day, but with the amount of lies and gaslighting going on, they can get stuffed.
Uh yea. On second reflection, hell no to Covid tests.
They do it by phone appt and telemed.
Oh Sadie, that was tragic.
Lin Wood is now on Gab.
https://gab.com/LLinWood
2,395 likes on his first post at the moment.

More info on Blood Clots caused by the Vaccines.
https://truthbasedmedia.com/2021/08/07/from-shots-to-clots-science-shows-covid-vaccines-cause-blood-clots/
Hmmm.
Maybe a meme “Shots Cause Clots” could be created/sent by folks talented at that….
https://twitter.com/4_04_Not_Found/status/1424103475500228610
We seem to be cutting in and out of the forum? Is that just me?
Yep.
404 error message from cPanel.
Doesn’t seem to be every page either. Could get an other post to load once.
Almost like the staff are rearranging the furniture again.
Same here 🙂 Right now all is well.
This is excellent. SIAP.
Don’t Get Jabbed: Powerful Video on “Killer Vaccine” that Needs to be Watched by Everyone
Watch here:
.
.
.
.
Reminder: this is from a FDA powerpoint presentation….
Good video. It’s downloadable, too, so at least some folks over here will be seeing it… thanks.
Lindell says Trump won New Hampshire:
Lindell TV
I’m seeing reports that he won a lot more than we know.
https://twitter.com/4_04_Not_Found/status/1424109683623796736
….. totally awesome DePat … 😉👍🇺🇸❤️🇺🇸 ..
L.A. county superior court system mandating vax.
JUDGES ARE EXEMPT !!!!
The nation’s largest trial court system announced on Friday that its 4,600 employees would be required to get vaccinated against coronavirus “as a condition of employment.” However, judges are not included.
The Los Angeles County Superior Court told staffers in a letter that they must be immunized “no later than 45 days after the Food & Drug Administration gives finals approval to at least one COVID-19 vaccine.” According to a recent report by The New York Times, the federal agency is expected to fully approve Pfizer-BioNTech’s vaccine “by the start of next month.”
https://citizenfreepress.com/breaking/employees-must-get-vaxxed-but-judges-are-exempt/
ALL OF THEM need to walk off the job NOW. That needs to happen with every mandate to make it stop!
Its ridiculous. Judges exempt for political reasons, like congress exempted themselves from obamacare.
Which makes them unfit to be judges!
May the Perfect JUDGE above ALL Judges remind them of their duty, and in the sternest terms.
A M E N … 🙏🏼 … 🤨 …
They are unfit since they refuse to comply with their own official order. I hope theres a walkout.
They are hypocrites. Therefore they are unfit for ANY office… One who flouts the law can neither enforce nor interpret (judge) the law…
Yes.
Another Dr. Sounds the alarm bell.
https://rumble.com/vkopys-a-pathologist-summary-of-what-these-jabs-do-to-the-brain-and-other-organs.html
It’s getting to the point where I don’t want to see or hear these reports because I’m so concerned about those I know who have been vaxed. 😟
Yet, we can’t look away.
You should think about it in the sense that you will understand this is vaxx injury and not organic. And, maybe help catch early symptoms.
… me too TheseTruths … 🥺😔 …
It’s the damn indoctrination of the young adults from elementary school through the college … they’re sheep 🐑 … 🥺
And yet —
This SAME Dr. Cole, after making it clear that the “vaccines” aren’t safe, that getting them is like “crossing the Rubicon”, that what the “vaccines” do to the body are “irreversible” — took the “vaccine” HIMSELF, and his FAMILY took the “vaccine” also.
After his presentation was criticized, Dr. Cole had an interview with in which he walked back somewhat on some things he said in his presentation. He made it clear that he’s not “anti-vax”, and emphasized that both he and his family were all “vaccinated.”
http://www.ktvb.com/articles/news/local/208/garden-city-doctor-reacts-fact-checking-his-statements-lawmakers/
Well, I’m “vaccinated,” too.
Against polio, smallpox, tetanus, measles, mumps and rubella.
I’d bet almost all of us are.
If someone asks simply “are you vaccinated,” the “yes, I am” is NOT A LIE.
Boom!
Love it.
You are not lying the so called jab is not a vaccination.
Correct!
🙂 We are on top of it
True, it should be referred to as vaXXX as poisonous medical porn.
That is a good one. OT had a video with the German lawyer . He had a Jewish woman as guest and she said paraphrased “this is like Nazi except this time the elite want to bring the Holocaust through the world.”
She said ” same people, Industry, Universities and medical just like in the Nazi time”. Why did Germans do nothing she said “because they were afraid”. “They put fear in us also “she said.
I cannot bring the video here or URL.
I could try but not sure people are interested.
Just tell me roughly where you found it and I can find the video to embed. This is exactly like my mother, who could not trust doctors. The one doctor lied and saved her life. A freak chance saved her.
You posted an URL from OT and in the comments someone posted the video.
I found it
The best response I saw to do you have the vax was “naturally”.
Nice.
I guess if someone is asking in earnest “I identify as vaccinated” won’t work.
But it would be hilarious. That counts, right?
And here we go… We are not doing Autopsies. Only 1 after 11,045 death.
Just crazy.
His first line is amazing. 11,000 deaths and the first autopsy now. “Is this science anymore?”
This is what I found so shocking back at CLIMATE CHANGE when there was no good proof of it and THEY JUST SHUT US DOWN.
I love that somebody called them “Fake Vaccines”. That really is what they seem to be.
Dr. Brian Tyson, MD 🇺🇸 (@btysonmd) Tweeted:
Leading Israeli Health Official: Vaccinated Account For 95% of Severe and 85-90% of New Covid Hospitalizations; Vaccine Effectiveness is “Really Fading” (VIDEO) https://t.co/Pj7Z1CGh4j
https://twitter.com/btysonmd/status/1424074713991507970?s=20
NOT TAKING THE VACCINE IS HOW TO SAVE GRANDMA.
Summary
So, we understand that corporations are, by necessity, public institutions. Commerce is necessarily a Public activity, taking place in the Public sphere. (May the Lord have mercy on any family that practices commerce amongst its members). In fact, the blending of Public and Private, the ongoing commodification of everything at the hands of Autonomy-worship and Capital, is a profound evil and one that must be opposed. Of course, it is the very people who most viciously defend “freedom” that are the ones plunging us head-first into a world of Might Makes Right wherein nothing escapes the world of politics and commerce. Is it at all surprising that the Left, those devote Autonomy-worshippers, are as hypercapitalistic as the neoliberals they claim to so thoroughly despise? (Hint: it shouldn’t be)
Once we understand that companies are not properly Private but are actually Public, we can begin to actually discuss how the Public sphere is one governed by the society’s morality. We must continue to understand that economics is embedded in politics which is embedded in ethics. There is no escaping the question of Good in society. “Autonomy”/”Freedom”/”Liberty”/”Self-responsibility” doesn’t cut it. “Harm” doesn’t cut it. “Consent” doesn’t cut it. It is time for a substantive moral outlook once again. Do not be confused by the Fabrication of “Freedom”: it is a recent phenomenon, and certainly a surmountable one. Not so long ago, we had our heads screwed on a little tighter. It’s time to get our sanity back.
.
https://apexsnotes.substack.com/p/there-is-no-such-thing-as-a-private
From Gettr:
I LOVE THESE!!!!!!!!
Politics makes strange bedfellows
BLM needs a rebranding to BLM-2.
Either way, step back and watch these passport cities begin to burn.
Also waiting for the one that says, “They got what they wanted from us and now are tossing us from the bus.”
They are MEMES
I very much doubt they are writen by BLM but since they are true, BLM is between a rock and a hard place. That is what makes them so great.
Memories of The Tuskegee Study of Untreated Syphilis in the Negro Male conducted between 1932 and 1972 by the United States Public Health Service (PHS) and the Centers for Disease Control and Prevention (CDC) is NOT going to make Blacks willing to trust the CDC this time around.
Over at .win (thedonald) they say 4Chan is responsible for this campaign.
Genius.
It’s very likely that Child Protective Services in States around the USA are gearing up for the young (under 18) to be taken custody for “their own safety”.
Article excerpt:
“For months, we’ve been warning people that Covid-19 “vaccine” totalitarianism would eventually lead to government taking our kids if we don’t let them get the jab. Well, it’s happening.
Dr. Robert Malone, the inventor of the very mRNA technology used in Pfizer and Moderna injections, was on with Steve Bannon on War Room today to explain the situation that’s unfolding in Boston, MA. A 14-year-old child who wants to be vaccinated could not get her parents’ permission, so the court has stepped in and revoked custody in order for her to get vaccinated without their consent.
“So, I’ve been called to testify as an expert witness in a court in Boston. The child who has been removed from their parents’ custody, a 14-year-old,” Malone said. “And the legal system is insisting on vaccinating this 14-year-old.”
Source: https://thelibertydaily.com/it-begins-boston-court-takes-custody-of-biotech-executives-14-year-old-child-to-force-vaccination-against-parents-wishes/
God rebuke them.
Democrats did this.
this.is.not.normal
Is this more work of the memesters? It’s GREAT.
everybody gets to meme!

OMG, that’s hilarious!!!
I legit read this sitting in a Subway eating lunch today, and I was laughing SO HARD people were staring like I had lost my mind!
It was great.
Remember, as Ashleigh Brilliant wrote,
“Of all the things I’ve lost, I miss my mind the most.”…. 😆
(Or, you could have looked to the right and said “I’m NOT crazy”, then looked to the left and said “and NEITHER AM I” )…
Haha! My reputation is bad enough around here!
That’s worthy of an O. Henry award… Brilliant…
I woke Hubby up I laughed so hard.
No words. Just none.
Oh, I have a great many, just none suitable for posting here.
I’m trying to make ”God rebuke them” my ultimate judgement vs. cursing at them.
Besides, no one can possibly outdo a rebuke from the Almighty. No. One.
This is true.
This worries me especially as I think it might be much bigger than just for one child. It could well be a (bogus) test case along the lines of those fomented by the SPLC, ACLU, and the rest of the gay mafia, where they put up a basically fake case to challenge existing mores and laws in order to push their depraved and wicked agenda.
In this case, it’s yet another attack on parental custody and authority and responsibility (before GOD) for their childrens’ upbringing. The so-called “UN declaration on the rights of the child” is the Devil’s tool underneath all of this.
And that idiotic court should well know that the USA is NOT a signatory to that “declaration”…
I hope and pray that this specious attempt to wrest the custody and future of that child (and other children) is utterly and completely quashed, either in this court, or the appellate courts.
Such a travesty and abrogation of justice and natural order cannot stand.
Maybe the Mohammedans think that nine years old is a suitable “age of consent”, but that was for Mo Ham Head’s “pleasure”. Who (or perhaps WHO) is benefitting from this?????
They want to STERILIZE the children PERIOD!!!
The UN Foundation DONATES to the CDC…
As Ted Turner, founder of CNN and the UN Foundation bluntly put it during an interview with Audubon magazine.
“A total population of 250-300 million people, a 95% decline from present levels, would be ideal.” – Source: The book You Don’t Say, by Fred Gielow, 1999, page 189.
“A massive campaign must be launched to de-develop the United States. De-development means bringing our economic system into line with the realities of ecology and the world resource situation.” – Paul Ehrlich, Professor of Population Studies His Co-author, Obama’s Science Czar, John P. Holdren and he wrote Ecoscience.
THE MALTUSIANS SPEAK….
“To achieve world government, it is necessary to remove from the minds of men their individualism, loyalty to family, tradition, national patriotism and religious dogmas…
…”The re-interpretation and eventually eradication of the concept of right and wrong which has been the basis of child training, the substitution of intelligent and rational thinking for faith in the certainties of old people, these are the belated objectives of practically all effective psychotherapy”. (Brock Chisholm, first Director General of the World Health Organisation)
”My three goals would be to reduce human population to about 100 million worldwide, destroy the industrial infrastructure and see wilderness, with its full complement of species, returning throughout the world.” — David Foreman, co-founder of Earth First!
100 million? Geesh, talk about moving the goal posts. It’s to be 500 million until this guy shows up.
Meanwhile the Senate’s 2,700 page, 1 trillion dollar Infrastructure bill that was voted out of committee with the help of 17 Republicans RINO’s address’s science denial. As a crime? Not sure, but you can bet it curbs free speech in some manner.
DAMN them to HELL!
BOSTON???
VERY BAD!
Just before I moved south a friend found his 12 year old was having sex with an adult and then he found the brother was having sex with his 10 year old. Both perverts were black.
He went after them on statutory rape. The COURT RULED THEY WERE IN ‘LOVE’ AND REMOVED THE GIRL (12) FROM HER PARENTS AND PUT HER IN AN APARTMENT WITH A PROSTITUTE…
THAT is the type of JUST-US you can expect in the Peoples Republic of Commiechusetts.
Hey all!
Question to the group, from a friend of mine who knows we are on it!
Is the Covid TEST, not the vaccine, but the test ITSELF, FDA approved?
Because if it is under an EUA, no one can be forced to take it. Nuremberg Code would forbid it.
So many people are being forced to either take the fake vax or be tested, and they don’t want to do EITHER thing.
So, do we know? Is the test FDA approved?
Good question since the CDC said it is not reliable due to not being able to distinguish “COVID” from previous viruses. That happened last Friday. Evening.
I believe I may have answered my own question!
Check out this page at the FDS:
https://www.fda.gov/medical-devices/emergency-use-authorizations-medical-devices/coronavirus-disease-2019-covid-19-emergency-use-authorizations-medical-devices
The diagnostics are under an EUA! They CAN’T make someone take the test without violating the Nuremberg Code!
Wow, there are a lot of people breaking that code.
I’m seriously thinking they don’t know what is in the Nuremberg code.
Well, most people don’t know how many judges are on the Supreme Court or who the vice-President is, so that wouldn’t be surprising.
Drop a tip to your favorite Gateway Pundit!
Ooooh, I think I will!
IMO they will run with it. Finger’s crossed!
I did it. We’ll see.
I guess you saw me on Gab, fangirling TGP, lol!
TGP is awesome! If I was a girl, I’d be fangirling them, too! 😉
where? on Gab? you get around!
Don’t tell anybody. 😉
Way to go, Aubergine!
Purple people feeder, as it were 🙂
Lol!
Here you go!
tips@thegatewaypundit.com
Excellent findings! I’ve never seen this quesiton come up before. They are conditioning us to not only reveal our medical information, but also to undergo whatever procedures they dictate. Well, no.
You bet they are! Asshats.
Great catch. The ASSHATS are on shakier ground than I thought!
This friend I have is wicked-smart and all over this stuff. She knows about all of you; I talk about what we do here all the time. She asked me to ask all my “excellent researcher friends.”
I love this place.
Truth is a good addiction! Jesus says!
Amen, brother Wolfmoon!
I found another page at the FDA about the EUAs for the tests.
THEY ARE ALL UNDER EUAs!
https://www.fda.gov/medical-devices/coronavirus-disease-2019-covid-19-emergency-use-authorizations-medical-devices/in-vitro-diagnostics-euas?ACSTrackingID=USCDC_2146-DM61940&ACSTrackingLabel=Lab%20Alert%3A%20Changes%20to%20CDC%20RT-PCR%20for%20SARS-CoV-2%20Testing&deliveryName=USCDC_2146-DM61940
We’ve got them, at least for now. They CAN’T force someone to take an emergency-use test.
HA! Excellent!
I think we win this round?
I think so. This is a huge scandal. The Demonrats are destroying America over BULLSHIT just to keep power.
Power is their religion. I think we have already established who their god is.
Oh lord, sorry, that link is LONG.
This page is longer! 😉
So kiddo has to get one to obtain medical care, his mri. So if i go to them, they’ll give me a blank stare. Even if i show them this and deny care. Then what?
Did they request this in writing?
I’m actually somewhat curious — let’s say he took a test and came out negative — so they do the MRI. But what would they do if he took the test and it came out positive? Deny him the MRI then?
And if they would do the MRI no matter the results of the test — what’s the point of the test?
No. I was told by the ordering MD and by the mri department. I can check online if the order states it. They dont give me paper copues.
Ok i just checked instructions. Neither the check out paper from the md which shows an order nor the online instructions state covid testing. What should i do?
I would show up as scheduled. If they ask for a test, say it wasn’t in the paperwork. If they want to test, say you want the FDA-approved test only, for obvious reasons. They can’t produce one, it doesn’t exist. Tell them they are NEGLIGENT if they don’t treat your son. Not HIS fault there is no FDA-approved test.
That’s what I’d do.
His test is under anesthesia, and we have to be there at 6 am. Which means up at 4 am to get there. Not as easy as just showing up.
I take it they’re sticking his head into the thing and are afraid he’s claustrophobic.
They are doing a brain and spine so keeping him still for an hr strapped down wouldnt be easy. I thought about it the last hr, and since this is really a back up in case his casting fails to r/o cerebral palsy and a couple other things, i may just cancel it altogether. 2 years ago Id be more inclined to do it, but the med profession has lost its mind in many ways.
That is so hard for a kid. I’m sorry.
Yeah, ty. If his legs respond ill cancel it. Ive got a few weeks.
I would ask them to recommend an FDA approved test. They are doctors. It is THEIR job to know this stuff.
I know how hard it is to confront medical people. But if we don’t push, they are going to kill us all, slowly. If I didn’t truly believe that, I wouldn’t say it.
I have to ask on Tuesday. Itll be an rnp who sees him for the next casting.
Good luck. I hope it goes well.
Thx.
At least it is just the test. A friend just got told her 11 year old daughter MUST have the VACCINE in order to receive medical care.
Wow. That is truly horrible.
As her if she wants molecular razor blades next to her daughter’s EGGS.
….and if that doesn’t click, try “molecular razor blades next to her potential grandchildren.”
Yup. Sometimes it pays to be direct about these things.
See next daily!
Friday night news dump.
That’s the way I saw it.
I so wish I had screen shot that page because I went back a few days later to capture the statement again. CDC changed it to omit the statement that the tests do not distinguish between COVID and flu.
Rather sure it is on one of QTree posts or even one of Wolf’s articles.
I might be able to find it. Do you have a web address for the page? Even as it is now?
I’m looking backward through my FB feed and got back to July 28th where I mentioned it regarding another post. That was not long after I first read it. Found it but have not reread the CDC material yet. I kept the whole URL since I wasn’t certain if what comes after the question mark matters in this context. https://www.cdc.gov/csels/dls/locs/2021/07-21-2021-lab-alert-Changes_CDC_RT-PCR_SARS-CoV-2_Testing_1.html?fbclid=IwAR2W3HLc2ggxsrS0ERq4c2CY1bl7x-vZxRmb-VorqFMag0dd3uYM0G8oHbA
I thought it was in the following quoted paragraph. There are clues… was the PCR test FDA approved or did it merely have a EUA? Further, is the PCR test multiplexed? “In preparation for this change, CDC recommends clinical laboratories and testing sites that have been using the CDC 2019-nCoV RT-PCR assay select and begin their transition to another FDA-authorized COVID-19 test. CDC encourages laboratories to consider adoption of a multiplexed method that can facilitate detection and differentiation of SARS-CoV-2 and influenza viruses.”
Here is the earliest archive of that page from the Wayback Machine. It is from July 22:
https://web.archive.org/web/20210722111802/https://www.cdc.gov/csels/dls/locs/2021/07-21-2021-lab-alert-Changes_CDC_RT-PCR_SARS-CoV-2_Testing_1.html
Thank you, Aubergine!! Wayback captured the page 229 times. I wonder how many times it was changed and what changed each time and date.
Gotta wonder.
A quick search turned up this:
https://celiafarber.substack.com/p/fda-rejects-pcr-test-for-approval
https://streetloc.com/page/view-news?id=1565
So the question is now whether the OTHER, REMAINING tests are FDA approved!
I really don’t think any of them are. Really.
Dunno the answer to Aubergines question.
But, what I distinctly recall from the March April 2020 time frame follows.
FauXi and scarf wench at pressers with Trump. FauXi and scarf wench were frequently talking about companies were allowed to roll out tests as they were developed. Streamlined process. I took THIS to be the same as NO CDC, FDA, NIH over sight. No formal process to approve the tests.
Good info! I sort of remember this, too. I hope Gateway Pundit jumps on this and runs!
This is a chilling article (check out the chart):
Jeremy James – “Vaccines” Target Christendom for Depopulation
https://www.henrymakow.com/2021/08/james-vaccines-target-chrstendom-for-depopulation.html
And the replacement population (illegals) are by and large not taking the vax.
How many weeks did we suffer through the global media’s narrative that India was being hit by the Wuhan virus that was renamed the Delta Variant and we were told of the massively overwhelming death numbers coming out of the large cities?
Here are the numbers from January 3, 2020 through August 2, 2021….
(Population #’s source – www dot Worldometers)
India with a population of 1,393,568,297
Recorded: 31,856,757 Confirmed Positive CASES….
Recorded: 426,754 Coronavirus Deaths
The United States with a population of 333,130,197.
Recorded: 36,498,830 Confirmed Positive CASES…
Recorded: 629,830 Coronavirus Deaths
In a Country with ONE BILLION Fewer in population, the United States has tested 5 Million more people infected with the virus and 200,000 more coronavirus deaths than in India, the country that was supposedly going to see MILLIONS die?
The numbers don’t support the narrative….
Corrupt CDC is why.
CDC = CHICOM DISEASE COLLABORATORS
:0) “corrupt” is not the word that I would use.
https://www.dailymail.co.uk/news/article-9870099/Retired-Marine-troops-plant-flag-Iwo-Jima-WWII-dies-aged-102.html
The first thought that comes to mind is may GOD Bless him and keep him.
The second though is, they don’t make them like that anymore.
RIP Commander Dave Severance. May we return to the freedom, bravery, valor, integrity, faith, and guts which characterized your generation… and hold that not just in our memories, but in our values and conduct…..
Just LOL. H/t Citrizen Free Press, the Dems who fled Texas are suing the governor because they are stressed.
https://www.dailymail.co.uk/news/article-9872097/Runaway-Texas-Democrats-file-lawsuit-against-Gov-Greg-Abbott-damaging-reputations.html
They were so giddy in that flight pic with no masks on, ready for their big adventure and vacation. Only a child would have been unable to predict that that would not last.
Can you spell p.e.r.s.o.n.a.l r.e.s.p.o.n.s.i.b.i.l.i.t.y?
This is simply PR.
The have a legal duty to be present when the TX congress is in session. If they did not, then the Abbott could not order their arrest to return them to the representative’s chamber to perform their duty (which he has).
This is a frivolous lawsuit made by leftist snowflakes pretending to be victims.
Hope they get SLAPPed upside the head (and everywhere else)…..
Unfortunately it is 25 to 70 years too late.
Yes, that’s why I’m laughing and talking about personal responsibility. They’re shirking their duty and trying to blame others. It’s juvenile.
They don’t need to see their families until they’ve performed their duties and as to seeing their families that is what visitation time in jail is for.
They’re getting off WAY too easy! How about treating them to the treatment of the January 6th political prisoners? Then they can talk about being victims and what they’ve been deprived of.
Yes, put them in jail and don’t give them a hearing for 7 months.
Good evening, q-tree friends. Just giving my two cents on pagination. I realize my situation is different and may not have much relevance, but I kind of prefer one page as I have a lot of trouble moving from one page to the next. I have to do it over and over, same, of course, if I want to go back to page one to finish reading or find new comments. The other thing I wish I could access is the yellow, as I don’t know which are new comments, so I end up rereading a lot looking for something I haven’t read yet, meaning newer comments.
Zoe, thanks for saying something.
I mentioned last night that I wasn’t crazy about the new “load more comments” feature but I will gladly live with it if that helps you out, and I would wager everyone else here would as well.
If I had the $$ this website would have a highly paid, full time team of IT programmers and experts to maintain and manage it, and I would direct them to create and manage features which would direct them to make things as easy for you as possible.
Again, thanks for saying something.
ForGodandCountry, you are so kind to say that, but I think many are prefering the pagination, and it’s not such a problem I can’t live with it, and it does have advantages, but I want you to know how much you are appreciating around this place. Also CTHULU and Wolf and many others have tried to help me with tech issues. So glad you came back after you were away for a while. Maybe some of us will meet someday at some event to celebrate our great country. See you around somewhere on pages 1 or 2 or 3 or 4. Lol.
Thirded. I prefer pagination. Pages are a hundred times easier to navigate.
If I can get through most of the first page as I go to it from my email, it’s not so bad, but if the email shows me the second page and I have only read half of the first page and want to get back to it, it’s hard but not the end of the world, and sometimes I can make it work if I keep trying. I think the vote is to go back to pagination, and I’m fine with that. Just saying. So wish I could see that hellow hilighter. Just doing a little whining. Lol.
I can live with going back to pagination. I think people want that, and I can understand. Just want to see that yellow hilighter. There just isn’t a way to make it easy for me to find new comments unless they are in reply to my own comments. Oh, well. Just whining.
The single page seems to work fine.
BUT the whole site is still acting wonky following the big shift a while back
And yet we have a lot to talk about.
date – # comments … 8/1 396, 8/2 867, 8/3 394, 8/4 770, 8/5 674, 8/6 658, 8/7 317 (and counting)
How to Speak Biden-ese: A Hilarious Lesson-
https://rumble.com/c/DineshDsouza
The Bidenese Libertine Army…
Good article.
CA GOP didnt endirse anyone, bc they wanted to endorse Kevin Faulconer, former san diego mayor and avid anti Trumper. Yeah THEY wanted HIM. But they had no way to hide it. Im shocked Larry Elder is out front but if he’s the front guy then I dont want the vote split.
Make L.A Great Again 🇺🇸 – RECALLGAVIN2020.COM (@lalovestrump) Tweeted:
Good … I was hoping they would not endorse a candidate
https://t.co/Vz6HnN2yGW
Won’t matter. We know who counts the votes.
Excellent video on mass psychosis.
https://twitter.com/FatEmperor/status/1424151061909147653?s=19
Sometimes I wonder if this COVIDiocy is the deception at the end of the age…
II Thessalonians 2:10-12:
You are not alone. Not hardly.
Revelations 13:16-17
Yes, this vaccine crap is grooming people to receive the Mark of the Beast.
What makes you think the Mark of the Beast would come in boxes saying, “Mark of the Beast, quantity 144 single-serve doses”?
Why wouldn’t it say “Pfizer-Biontech” on the box?
Yup. This could be it.
A perfect description of what’s happening. The more people fall for the propaganda, the more fearful they become and the less they care about principles and individual rights. I guess enough time has passed since WWII that people no longer understand about the mass deception/psychosis that occurred then.
I’ve been saying a few times about the need to pray, intensely and intently in these times where the demons and the DEMONRATS are doing their best to ruin our world and GOD’S Creation. Daniel faced many of the same issues, including being forced to do things and worship things which GOD had forbidden.
Here’s a spiritual that describes that, and Daniel’s response. Some great Bluegrass from Ricky Skaggs and Patty Loveless. Well worth listening to and reflecting upon…
P.S. Catch the Rebel Yell at 0:58, and their great response to it 🙂
P.P.S. The cadence at the end is really good, too…
P.P.P.S. OK, I know, cadences are usually at the end, but they did a really good job here…
Awesome video! Patty Loveless is one of my faves. Never knew what happened to her. Think I’m listening to the wrong stations.
Something is still wonky. I scroll through the entire post, turn all the yellows to white? and then later click the orange ball which has slowly incrementing. Then scroll again. Oddly most of the original yellows have returned to yellow, with a few that are new. I have a touch screen and my right thumb is going to be awesome.
I’ve been seeing whites turn to yellow for a few days now, and so I doubt it has anything to do with the pagination setting.
Maybe try Wisk?
Thanks! I’m watching all this and waiting to ask the question – stay with “LOAD MORE” or return to pagination. Soon. Opinions rendered prior to that time will be noted, but if changed, no problem.
DId you see my reply to barkerjim?
The white posts turning back to yellow has been going on for a few days.
Yes! I’ve seen that, too.
I believe that it’s due to client data (the wiggly bars) not making it back to the server.
I’m good with a single page per post.
Did you see the stats I posted earlier on # of comments per open?
I saw, but did not stop to analyze yet. If you have the BLUF, it could save me time!
date – # comments …
8/1 396,
8/2 867,
8/3 394,
8/4 770,
8/5 674,
8/6 658,
8/7 492
Longer than that.
If you saw what Zoe wrote, and For God & Country’s response, I think your mind might be made up.
Thanks – I had not seen that. Helpful to see that two more people are good with it.
Based on all the responses, I’m leaning toward staying with single-page as the lesser of two minor evils, but If we stay there, it will be for the good of the site.
Part of Wolf’s security model is absolute resistance to sympathetic minorities whose needs violate the security model. I’ve ignored Zoe’s needs on other things that conflicted with the mission, and I will always make decisions based on the needs of all of us.
But given all that, I was trained for this job as a kid, and I know all about the value of a blind team member.
I’m glad you are a hardass. We need that here. Usually I am, too. I had a weak moment. Yesterday was a long day.
I don’t get a “load more” option. All the new posts are loading and the newest ones that are not replies to others appear at the top. I didn’t know what people meant by “load more” until I went to yesterday’s daily to look something up, and then I saw it.
Yeah, it’s interesting how that works!
Reposting this.
Btw, this guy is running for Gov. of Texas.
And I have a new candidate to support.
.
He is excellent. He speaks at a fast rate, which shows that he speaks from the heart and knows his subject matter.
A few of his points:
Kiddo had rsv pretty bad. I believe others here had kids with it.
Just be aware, for your grandkids. It dies have a test to diagnose.
Alex Berenson (@AlexBerenson) Tweeted:
So we’re all clear: when you read those worrying stories about a respiratory virus filling children’s hospitals, you are reading about RSV. And the likely reason this is happening now is because lockdowns prevented normal exposure, so 18 months of cases are happening at once: https://t.co/c1acIYeGCT
Children wearing masks likely contributed to this. Or so MeThinks.
There is so much garbage 9n those masks. I dont know where mine got it, wasnt in preschool yet but trip to the er and some serious meds. Luckily he responded to extra doses or they would’ve flown him to kids hospital.
Kids sneezes, coughs, and boogers are copious. Keeping it on you hands and faces is bad news.
So THAT’S why they want to deploy a CLOT SHOT for that one, too. To get the kids.
Yep. Wolf, i think they will try to force this on kids at in person school….like sex ed, abortions, trans bs….
Paging our WA and OR Peeps.
Wondering what Covid lunacy I may encounter next week, as I trek to Glacier National Park.
Oregon – Eastern side US 95N & I-84N.
Washington – Eastern side US 395N & I-90E. Overnight in Kennewick. Transit Spokane, not stop.
Montana – Normal, I am guessing. Traveling through Glacier, then I-15S
Idaho – A nothing burger short of liberal enclaves like Twin Falls.
Nevada – Rural Nevada blowing Sisolak off.
Did not wear a mask anywhere in Spokane, ID (Twin Falls), MT. Just don’t read any signs. No one enforcing anyway.
Thanks. Good to read. Yea, wherever signs may be up, I ignore. If they ever insist, I leave.
Don’t run low on fuel.
So true. A logistics guy here. Pretty much know where I am fueling with plenty left in the tank each time. Hotels are locked on. Weather in 15 day forecasts looking good. Always carry a reasonable amount of what if stuff, for when Murphy shows up.
My son asked me “since when do Americans read signs?”
You’ll be on the conservative side of the mountains of OR and WA, so that means more common sense will prevail. However, I live in Salem, and hardly anyone is still wearing masks here. I haven’t heard if our idiot governor has reinstated masks and I’m not going to look because I don’t care. I won’t be wearing one.
Perfect. Thanks.
Newsom is too scared of his recall to call for a statewide mask re-mandate, but the local county health officials are (I know it strains belief) even bigger idiots.
And it’s coming through while my car is being repaired (I never used a mask with the mechanic, but it meant that I couldn’t easily just move to another store), my refrigerator is going into overload again, and the fiancee is acting up….
Montana is about how it has been all along. One or two stores here in my small town are back to masking employees, but very few customers are wearing them. Avoid Missoula, if possible, they are liberal lunatics there.
You sneezed. Congrats you have covid.
Kyle Becker (@kylenabecker) Tweeted:
‘The Apocalypse shall come as a case of the sniffles’
https://t.co/mBam3BfWfs
I just checked in on Selwyn Duke. “Longtime Physician on COVID Care: “When Did the World Become Insane?” https://selwynduke.typepad.com/.a/6a00e54eeb14318834026bdee68e0c200c-120wi
By Selwyn Duke He’s fed up and, he says, must finally speak out beyond his private circles even if it means being canceled. What bothers Dr. Matt Bettag, a physician with 24-years experience, so much is that he has “never before seen the medical establishment just stop thinking,” as he puts it. “Insanity is the new rule,” he continues, “and common sense cannot even be discussed.”
Bettag, who has been a practicing ENT for 19 years of his career, is, of course, referring to COVID craziness, to schizoid prescriptions that mutate faster than a virus ever could.”
https://thenewamerican.com/longtime-physician-on-covid-care-when-did-the-world-become-insane/
I think more and more people are seeing the insanity.
I agree, I think more people are seeing it. Hopefully, that helps us all.
Wolfie, Q Treepers,
Do you realize the the Covid Genocide Weapon is BACKFIRING!
Who is taking the Jab?
Democrat brainwashed Sheeple.
WHO IS REFUSING THE JAB?
Conservative Rebels
As long as Conservatives hold their ground and yell NO GENOCIDE JAB! they will get increasingly frantic.
Meanwhile the whole mess is unraveling as more and more people are starting to see the truth about the Genocide Jab.
..
Agreed. The Democrats are going literally insane. They are upending society.
HOLD THE LINE.
STAND YOUR GROUND.
THIS IS THE HILL.
In any dictatorship takeover, the despots kill their own followers first.
When all is revealed, they are going to panic like we’ve never seen before.
A tranny.
Who worked for Piglosi AND Hillary.
Ttried to lure a 14 y/o for sex.
Not holding my breath until those two denounce his actions. 😮💨
They wont.
Love this lady!!!
Clearly I live in the wrong congressional district.
So for forty years we are fed a diet that has made 70% of us over fat. 30% obese. Then they infect us with a virus that targets the obese. And then they wonder why we don’t trust them.
Yeah it just barely broke 500 I think sometime on Sunday.
[…] Part XIII – Rutherford On A Roll […]