What is it that feeds our battle, yet starves our victory?
Speaker Johnson: A Reminder.
And MTG is there to help make it stick.
January 6 tapes. A good start…but then nothing.
Were you just hoping we’d be distracted by the first set and not notice?
Are you THAT kind of “Republican”?
Are you Kevin McCarthy lite?
What are you waiting for?
I have a personal interest in this issue.
And if you aren’t…what the hell is wrong with you?
Fun Quote
(HT Aubergine)
This is amazing. This is glorious. Summon a surgeon – it’s been a little over a week and you’re supposed to call the doctor after just four hours.
From Kurt Schlichter, who can certainly write a good rant (https://townhall.com/columnists/kurtschlichter/2025/01/30/trumps-winning-streak-is-totally-discombobulating-the-democrats-n2651308)
Yep, Kurt has noticed that lots of people are getting twanging schadenböners.
And you do not have to be male to get this kind of böner.
Hat tip to Scott (I think–if it wasn’t Scott it was 4GodAndCountry) for this video, which implies a LOT of schadenböners in our future.
Lawyer Appeasement Section
OK now for the fine print.
This is the WQTH Daily Thread. You know the drill. There’s no Poltical correctness, but civility is a requirement. There are Important Guidelines, here, with an addendum on 20191110.
We have a new board – called The U Tree – where people can take each other to the woodshed without fear of censorship or moderation.
And remember Wheatie’s Rules:
1. No food fights
2. No running with scissors.
3. If you bring snacks, bring enough for everyone.
4. Zeroth rule of gun safety: Don’t let the government get your guns.
5. Rule one of gun safety: The gun is always loaded.
5a. If you actually want the gun to be loaded, like because you’re checking out a bump in the night, then it’s empty.
6. Rule two of gun safety: Never point the gun at anything you’re not willing to destroy.
7. Rule three: Keep your finger off the trigger until ready to fire.
8. Rule the fourth: Be sure of your target and what is behind it.
(Hmm a few extras seem to have crept in.)
Spot (i.e., paper) Prices
Last week:
Gold $2,858.10
Silver $31.20
Platinum $953.00
Palladium $945.00
Rhodium $5,100.00
FRNSI* 137.261-
Gold:Silver 91.606-
This week, 3PM Mountain Time, Kitco “ask” prices. Markets have closed for the weekend.
Gold $2,911.50
Silver $32.60
Platinum $974.00
Palladium $934.00
Rhodium $6,000.00
FRNSI* 139.844-
Gold:Silver 89.310-
Palladium is below platinum again…but look at rhodium, which has gone up nine hundred bucks!
The people who bloviate on this sort of thing for a living (if this is all I did I’d starve to death) claim the precious metals are “consolidating” with gold in the 2910-2920 range while the stock indices go down. At least silver is up relative to gold!
*The SteveInCO Federal Reserve Note Suckage Index (FRNSI) is a measure of how much the dollar has inflated. It’s the ratio of the current price of gold, to the number of dollars an ounce of fine gold made up when the dollar was defined as 25.8 grains of 0.900 gold. That worked out to an ounce being $20.67+71/387 of a cent. (Note gold wasn’t worth this much back then, thus much gold was $20.67 71/387ths. It’s a subtle distinction. One ounce of gold wasn’t worth $20.67 back then, it was $20.67.) Once this ratio is computed, 1 is subtracted from it so that the number is zero when the dollar is at its proper value, indicating zero suckage.
Latest Flerfer Goofiness
OK unlike last week I’m going to try to supply some actual content. (Last week was nonstop busy.)
Our friend Fkatzoid is back. Watch him duck and weave when he’s asked where the south pole is (at 16:45).
Will Duffy is trying to make the point that whether you head south from Africa, South America, or New Zealand, you end up at the same place when you get to the south pole. According to the Gleasons’s Map, however, the South Pole isn’t a point, it’s a circle approximately 60,000 miles in circumference, so that shouldn’t happen. Either Fkatzoid is an even bigger idiot than he showed himself to be last time, or he’s trying very hard to evade having this pointed out to his audience.
Later on at about 2:31:45…apparently Lisbeth (who went to Antarctica) is on the verge of joining Mark Sargent’s channel; MC Toon begs her not to ruin her life doing so.
Just a few minutes later, you see someone named JK trying to find a video proving that people who try to go to Antarctica will be intercepted by any of a number of different navies and turned back as soon as they sail across 60 S latitude. He claims there are many of these videos; he eventually finds the one Will Duffy expected–taken in the Bass Strait between Tasmania and mainland Australia, nowhere near Antarctica. Listen to McToon’s rant at 2:45:22.
Here’s another debate with Duffy destroying someone I’ve never heard of named Nathan Thompson. (Not to be confused with Nathan “where are the guns” Oakley.)
Two Birds…One Stone
OK this one is going to seem like geology…then physics…then back to geology. It’s a good illustration of how all of human knowledge about the natural world is interconnected. Sometimes great progress is made when people in two different fields get together; sometimes a new discipline even is formed–recent work has done much to highlight the effects of living organisms on the geology of the Earth…yes, our rocks would be different if there were no life on earth (and there’d be no geologists to notice, of course).
An Extremely Inadequate Intro to Mineralogy
Let’s take a very brief and incomplete (and likely incompetent, as I am out over my skis here) look at mineralogy.
I’ve talked about rocks a lot but not so much about what they’re made of. If you look closely–perhaps it will take a microscope–at an igneous rock (one that cooled from the molten state) you’ll see it’s made up of a bunch of different kinds of crystals. Crystals form when a chemical compound comes out of solution and the individual molecules line up in a regular array.
Some rocks are just one big crystal. Others are multiple crystals of the same thing.
The compounds that make the crystals are minerals (and one of their characteristics is how the crystals are shaped).
What are those compounds? Let’s set the stage a bit. If you take the outer layer of the Earth, the Earth’s crust, and analyze a completely average piece of it…it’s 46.1 percent oxygen by weight. Oxygen! There is much, much, much more oxygen beneath your feet than above your head in the air. Oxygen is also the third most abundant element in the universe as a whole–after hydrogen and helium.
Coming in second at 28.2 percent is silicon. Then aluminum at 8.23%, iron at 5.63%, calcium at 4.15%, sodium at 2.36%, magnesium at 2.33% potassium at 2.09%, titanium at 0.565%…and everything else is at 1/7th of a percent or less. At the bottom end you have rhenium at 7/10ths of one part per billion. (However two gases, krypton and xenon, also show up at even lower percentages, and a bunch of transient radioactive elements are lower still than that.)
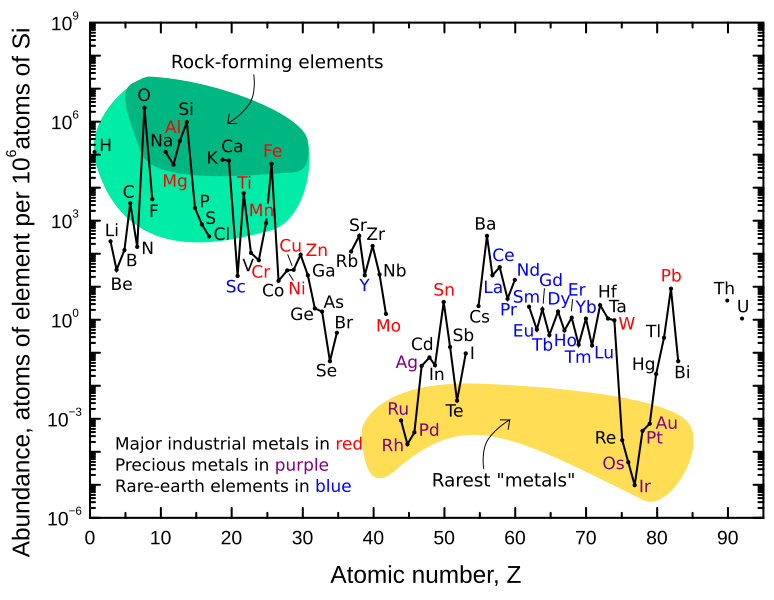
The ones at the top of the list don’t ever show up in pure elemental or “native” form; they’re pretty reactive. Minerals will be largely (but very luckily for us, not completely) formed of these elements.
The elements in general are divided into groups according to the “Goldschmidt Classification.” The groups are “lithophile” (rock loving), “Siderophile” (iron loving), “chalcophile” (bronze loving), and “atmophile” (atmosphere loving). The group an element is in is a huge determiner of its fate. Lithophile and chalcophile elements both appear predominantly near the Earth’s surface, in the crust; with the chalcophile elements often combining with sulfur. Siderophile elements largely sank, with almost all of the iron, towards the Earth’s core.
(There is a very slick wikipedia graphic for this, a periodic table colored by Goldschmidt classification…but it’s actually a table rather than an image and I was unsuccessful in getting it copied over here. Link: https://en.wikipedia.org/wiki/Goldschmidt_classification )
There are officially 6,118 mineral species known to man today. Minerals must be naturally occurring and forming by natural or geological processes, must be a solid substance (with the exception of native mercury). Water and carbon dioxide are not minerals even when they show up embedded in rocks, but water ice in glaciers is a mineral. A mineral must have a well defined crystal structure. (This ends up excluding things like obsidian which don’t have a crystal structure.) And the chemical composition must be well defined. However, that could include mixtures of similar compounds; sometimes one element will substitute for another of similar size and chemistry to one extent or another.
There are a number of different ways minerals can be classified, based on hardness, color, crystalline structure, cleavage (i.e. which planes it will split on most cleanly), specific gravity (galena, a lead ore, is very dense, for instance–over seven times that of water whereas the typical rock is in the 2.5-3.5 range)…and by chemistry. But this is far from straightforward, since nothing is pure. For instance a mineral whose structure is largely silicon will often have an aluminum atom substituted for the silicon; sometimes this is a regular substitution, making a distinct chemical series.
Minerals fall into a number of different groups; the most common by far is silicates; these are minerals formed by different arrangements of the [SiO4]4- tetrahedron, one silicon atom surrounded by four oxygen atoms.
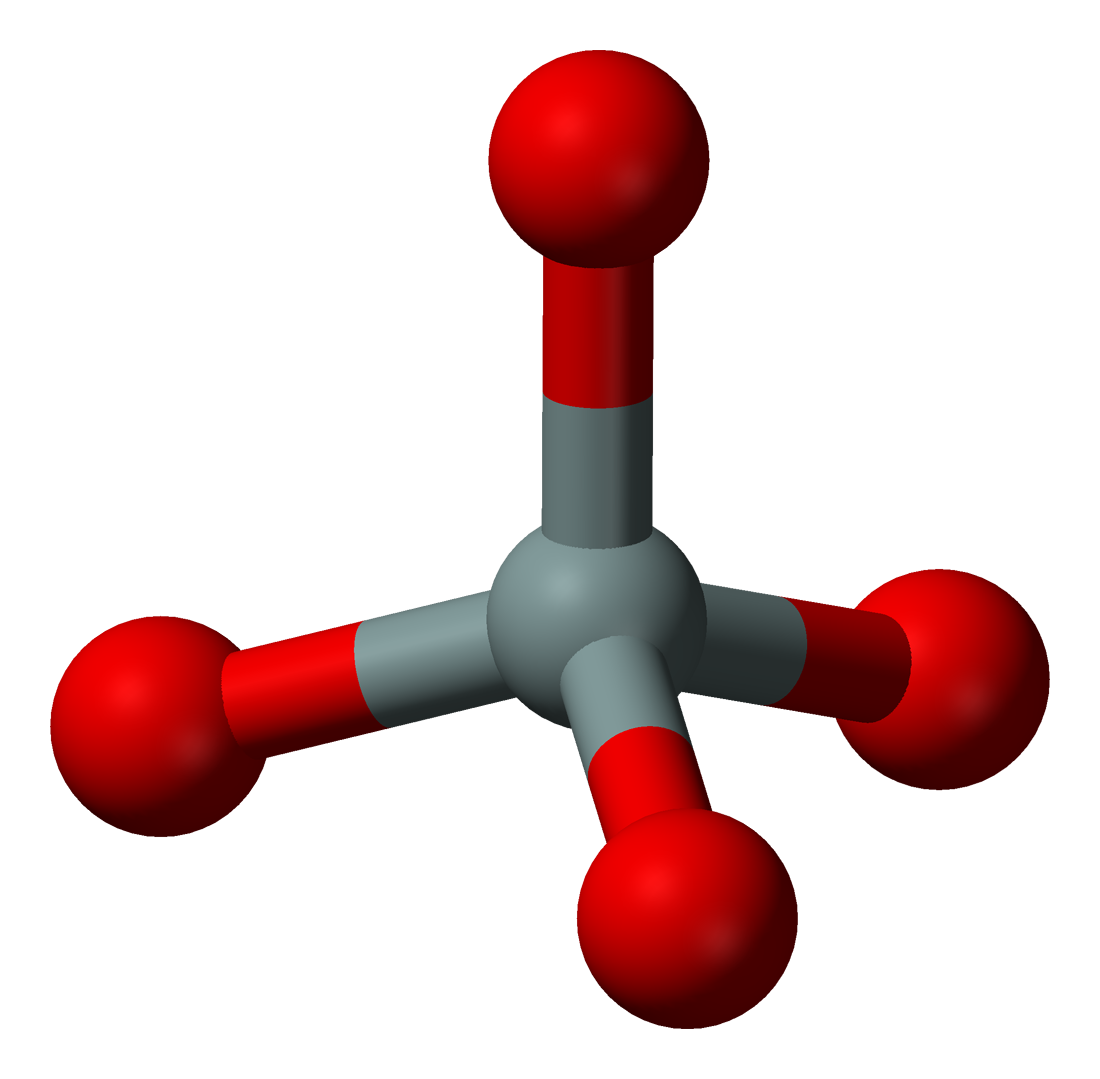
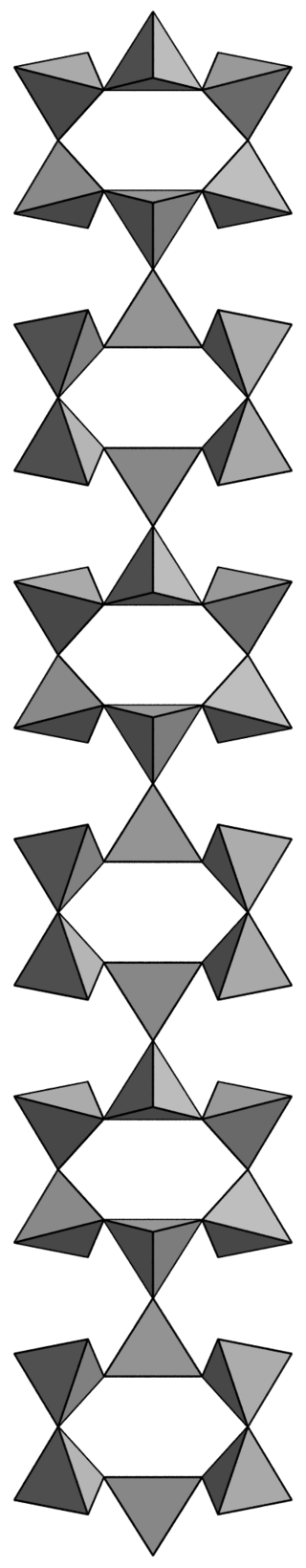
This shouldn’t be too much of a surprise, after all oxygen and silicon make up most of rocks. There are a simply staggering number of ways to combine these tetrahedra at the corners (where an oxygen atom will end being shared by two tetrahedra); chains, rings, lattices…just for instance:
The most basic of these is quartz, consisting of nothing but silicon and oxygen. Since each oxygen atom is shared by two silicon atoms, the formula ends up being SiO2.
Quartz, when absolutely pure, is clear as glass. Different impurities will give it colors, smoky quartz and amethyst being examples, but there are many more.
And in some cases other elements are interspersed with the tetrahedra, or sometimes the silicon is partially replaced by other elements. This can alter the structure as well as the composition.
The most common of the silicates are a grouping called the feldspars, where Al3+ substitutes for the Si4+, but this creates a charge imbalance that requires other elements added in as cations. You end up with [AlSi3O8]– or [Al2Si2O8]2-. In other cases silicates can form in sheets, like mica.
If you haven’t realized this by now, it turns out that silicates are bewilderingly complex. For a deep dive: https://en.wikipedia.org/wiki/Silicate_mineral.
In other groups we have native elements. For example gold, silver, and copper appear in native form as nuggets. There are also platinum nuggets. But also there are diamonds and graphite, both forms of native carbon. Sulfur also appears near volcanic vents. And in many cases the nuggets aren’t pure but are alloys, but still a lump of metal, rather than a “rock.”
Next we have sulfides, compounds of metals and sulfur, famously iron pyrite (fools gold), red cinnabar (a mercury ore). Sometimes tellurium, arsenic, or selenium will substitute for some or even all of the sulfur.
Oxides are metals combined with oxygen, such as hematite (iron), bauxites (aluminum), magnetite (iron again).
Halides are those where a halogen (fluorine, chlorine, bromine, iodine) is the main anion; table salt is the most common example, with chlorine combined with sodium.
Carbonate minerals have a carbonate [CO3]2- group in them. They will react with acids; so field geologists will often have a small vial of acid to test for them. The most common is calcium carbonate…also known as calcite, the main component of limestone. This is weakly soluble in water, leading to the formation of cave systems.
There are sulfates (distinct from sulfides mentioned above) with the sulfate anion, [SO4]2-, combined with something else.
The last common group is the phosphates, with a [PO4]3- unit, combined with something else. These minerals are what our bones and teeth are made out of.
It’s a gigantic mess, honestly; and it gets more and more complicated when it turns out that a mineral can be a mixture of, say, two different sulfates mixed together; the formula ends up including a bracket with two or three different atoms specified because they are intermixed in some proportion.
I have not even scratched the surface of this topic (and those familiar with hardness testing will see the pun). I may not have said anything wrong in this section, but even if we’re that fortunate, I’m sure a real mineralogist would find much to complain about, important things left out, inconsistent “depth” of the dives I took, and so on. I know I said next to nothing about crystal structure and I may try to rectify that some day.
Back to the Historical Narrative
OK so back to the story: By the mid 1800s geology had made huge strides to systematize the variety of rocks and landscapes we see here on Earth. Geologists had even developed the ability to describe what had happened in the past in some arbitrarily picked location. Glaciers, lakes, oceans, desert…all had left telltale signs in the rock. They saw a world of mostly slow change…but with the occasional disasters, local in scope not worldwide.
They had even realized the Earth must be far older than previously thought; the events they could read in the rocks simply could not have happened fast enough to fit within a few thousand years of time.
Impressive work. There were obviously a lot of unsolved problems (like how it could be possible that former sea floor bottom ended up high in the Alps), but still a lot learned.
Physics and astronomy (closely associated with each other) were pretty much the most successful and advanced branches of scientific knowledge. Were astronomers and physicists at least somewhat impressed with what geologists had come up with?
Perhaps but in one key respect the answer was probably more like, “You gotta be shitting me.”
You see the physicists and astronomers of the mid 1800s couldn’t possibly see how the Sun could be old; if the Sun weren’t old neither could the Earth be old. There was simply no way to power the sun for those lengths of time. However the geologic evidence was simply overwhelming.
Beyond suspecting that geologists were smoking something that was distinctly not a mineral (and vice versa from the geologists’ point of view), there was little that could be done. Tons of hard evidence (i.e., rocks) vs. quite well established kinetics and thermal physics. Neither of them could be shaken.
So what was the cause of this disconnect?
In 1862, William Thomson (1st Baron Kelvin…after whom the Kelvin scale is named), published calculations that assumed the Earth had started out completely molten, then computed how long it would take to cool to what we see today. His answer was 20 – 400 million years. OK, that seems a bit low to geologists, but not horrifically so. (He did not account for convection inside the Earth, which would increase the number…nor for other factors he simply couldn’t have imagined, which I’ll get to.)
The big problem was that he also computed how long the sun could have been shining at its present brightness, if it derived all of its energy from gravitational contraction. And that answer was 20 million years. It agreed with his Earth calculation at the low end so that made sense–Thomson probably reasoned that the Earth therefore had to be 20 million years old, but geologists (and biologists) simply couldn’t believe the Earth was that young. Other physicists (Hermann von Helmholz and Simon Newcomb) got similar values of 22 and 18 million years, respectively.
Other possible sources of solar energy were combustion and impacting comets and asteroids. The first was ridiculous. If the entire Sun, huge as it is, were a burning pile of coal, it would be gone within a couple of thousand years at the rate it would have to be burning to be as luminous as it is. This is not even long enough to carry us from the Great Pyramid to Julius Caesar, much less to today. Asteroid impacts sounds more promising, until one realizes there’d have to be so many of them that surely Earth would be catching a lot more of them than we actually are getting. And it was only good enough for a few hundred thousand years. The other flaw was that the sun would be increasing with size as more matter accumulated in it, and that imposed a strict time limit too…after a certain amount of time the Sun would simply be bigger than we see it.
Another tack taken by physicists and astronomers was to use the moon. George H. Darwin (son of Charles “the” Darwin) was an astronomer, figured out that if the Earth and Moon had split apart while still molten, tidal forces would have created our current situation with a 24 hour day after 56 million years. This may not look like it to you, but given the sorts of approximations that both Darwin’s and Kelvin’s calculations enailed, that’s actually close enough to Kelvin’s number that it appeared that both of them were likely on the right track. (When two totally different methods of computation give similar answers, that’s a powerful argument that the actual answer is pretty close to the ones we computed.) Yet another tack, computing how long it took for the oceans to accumulate the salt they contain, based on erosion of rocks, gave an answer of 80-100 million years for the age of the oceans.
When you see an apparent contradiction like this, something is missing from your mental picture. Or perhaps you have a wrong premise. Because an actual contradiction cannot exist.
And, as it turned out, one mineral, when it was discovered, turned out to be the beginning of the path not just to resolving this, but fulfilling another thing that was on the geologists’ wish list–one they never thought they’d get. Like the kid who doesn’t bother asking Santa for the really expensive toy for Christmas…but Santa read his mind and he gets it.
The mineral is an oxide, one called pitchblende. This was first described in 1772 by F. E. Brueckmann. In 1789 M. Klaproth worked with this stuff and discovered the element uranium.
[Uranium oxide has been in mosaic glass from Roman times; clearly they’d found some of the ore and experimented to see what it would do to color glass. However, we don’t have written records of the Romans recognizing it as a distinct material.]
Here’s some nice big crystals of pitchblende:

Uranium was nothing special, just another of a bunch of metals being discovered around this time. Along with such other favorites as cobalt, nickel, manganese, tungsten, niobium, tantalum, and chromium. Curiously, pitchblende also includes some lead, without fail. No such thing as “pure” uranium oxide pitchblende. That seems kind of weird because lead and uranium are chemically quite different.
Flawed analyses led to uranium’s atomic weight being calculated at 156 or so; later on the mistake was realized and the atomic weight was corrected to 238, far above anything else known at the time. Kind of interesting to geeks; no one else cared.
In 1895 this changed. And so did the world.
Henri Becquerel was trying to see if uranium salts, known to fluoresce in visible light, also fluoresced in X ray frequences. (X Rays had been discovered the year before by Röntgen.) [As a reminder, fluorescent things will glow in bright colors for a while after being exposed to ultraviolet light. This can actually be used to identify some minerals. Becquerel wanted to see if they also emitted X-rays alongside the visible light.] He’d expose the compounds to sunlight, then set them next to wrapped photographic plates. If the plates fogged, he would conclude the uranium compounds were giving off X-rays after being “charged” by the sun. Then he had days of cloudy weather, so he put the uranium salts and wrapped plates in a drawer while he awaited sunny days. Ultimately he decided, what the Hell, and developed the plates without exposing the uranium salts to sunlight, and found that they had fogged anyway. Well this was new!! Further experimentation established that uranium emitted strong radiation, all the time, no matter what.
Even more experimentation established that the uranium was turning into lead as it did this. Which is why pitchblende always has some lead in it, even though lead is very different from uranium, chemically.
This led to our current picture of the structure of an atom–which up to then had not been proven to exist. (The final piece of proof was supplied by Albert Einstein in 1905, the Annus Mirabilis)
That is a very long story. Detailed here (9 – End of Classical Physics (Rays & Radiation)):
And here (13 – Ernest Rutherford):
And here (17 – Nuclear Physics Finds a Hammer):
And here (19 – Antimatter):
And here (20 – The Little Neutral One (Neutrinos)):
One key thing to note is that this new “radioactivity” was extremely energy intense, far more so than burning coal, and now we had a hints of a power source that would allow the sun to shine for hundreds of millions–even BILLIONS–of years.
And this is indeed the case, as described here (22 – Powering Stars):
And the world was never the same, because this ultimately led to nuclear weapons.
But for our purposes here, the main effect is that now there was no more contradiction about how old the Earth might be. The Sun could indeed be old enough for an old Earth.
And Now We Can Measure It
Surprise! We also now had a way to measure the age of some rocks, to put actual numbers on things.
To explain this adequately (given the fact that there are charlatans out there who try to fling mud on this, and some of you believe them), I’m going to try to do a Science For Senators review of radioactivity and nuclei. It’s a bit densely packed since I’m not telling a story here. (The story was in all those posts above.)
Matter is made up of atoms, very roughly a hundred picometers (a picometer is a trillionth of a meter) across. Most of this volume is taken up by electrons (which have a negative electrical charge) that are bound to a positively charged nucleus (plural, nuclei). The nucleus contains almost all of the mass of the atom yet occupies a space only a few femtometers (a femtometer is a quadrillionth of a meter across); roughly 1/10,000th the diameter of the atom as a whole.
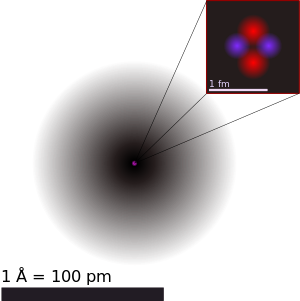
The nucleus, in turn consists of protons–positively charged particles–and neutrons–neutrally charged particles. Other than the charge, these two particles are very similar to each other–the neutron is just a bit more massive–and they’re collectively referred to as nucleons. (Neutrons are blue, protons red in the diagram below…but they don’t actually have color and they’re not actually shaped like little hard spheres, so the diagram is notional.)

As it turns out the number of protons in a nucleus (the “atomic number”) determines what chemical element it is. One proton: hydrogen. Six: carbon. Eight: oxygen. Twenty-six: iron. Forty-seven: silver. Seventy-nine: gold. Eighty-two: lead. Ninety-two: uranium. (Plus all of the other numbers in between of course.) In order to balance out, an atom will have the same number of electrons as protons, at least until it starts sharing or even giving or taking electrons with, to, or from other atoms–which is what chemistry is all about.
The number of neutrons, on the other hand can vary, even within an element. Just for instance, most uranium nuclei have 146 neutrons in them, but some have only 143. This has very little effect on the chemistry, but it is possible to very painstakingly sort these out. The two different types of uranium are described by their mass numbers, the total number of nucleons. 92+146=238, and 92+143=235; uranium-238 and uranium-235, respectively. These different-weight forms of the same element are called isotopes.
As it turns out radioactivity, when it happens, happens to nuclei. There are two main kinds of radioactivity that matter for our purposes here, alpha decay and beta decay.
Alpha decay is when a large nucleus basically pukes up a helium nucleus (containing two protons and two neutrons–mass number of 4). Since the nucleus gives up two protons in doing this, it changes to another element; this should therefore happen five times as uranium turns to lead, changing the atomic number from 92 to 82. Except that that’s not actually right; it turns out to be eight times. That’s because uranium-238 is becoming lead-206; that’s a difference of 32 mass units and eight alpha decays does that.
The reason the atomic number changes by ten rather than 16 (two per alpha decay) is that there is also beta decay. In this kind of radioactive decay, a neutron turns into a proton, ejecting an electron (which flies off into the distance, so you can basically forget about it) and a neutrino (which flies off away forever, so you can really forget about it). The effect is to leave the mass number unchanged…but it increases the atomic number by one (we have one more proton than we used to). To make up the discrepancy noted above, uranium, in turning to lead, must undergo six beta decays.
Technically speaking what I just described is negative beta decay, because it spits out a negatively charged particle. The reason why one might to be anal about this is that there’s actually a different kind of beta decay that may come into play, though…and that’s positive beta decay, where a proton spits out an anti-electron (“positron”–yes, this is antimatter) and turns into a neutron (the exact opposite change from the first kind of beta decay). This causes the nucleus to go down one in atomic number, again without changing the mass number.
Uranium and thorium (atomic number 90) undergo alpha decay, as do a lot of the things they turn into on the way to becoming lead (as do many of the intermediate elements in between and on the way). A lot of the intermediate products undergo beta decay. That’s all stuff at the high end of the periodic table, though.
It turns out that a lot of much lighter elements…ones we thing of as stable…are at least partially made up of isotopes that do one or the other form of beta decay (there are dozens of examples). Even potassium has a long-lived isotope (potassium-40 or 40K) that decays, in fact it can decay two different ways: negative beta decay or “electron capture” where a proton absorbs an electron. The first turns it into calcium-40, the second turns it into argon-40.
Our atmosphere is about one percent argon, and that argon is almost all argon-40. The sun’s argon–which presumably came from the nebula that condensed to form the solar system–is almost all argon-36, which leads to the conclusion that none of the Earth’s original argon is still around, and all of the argon in the atmosphere is actually from the decay of potassium-40.
There is just one thing I haven’t mentioned yet. Alpha and beta decay occur at constant rates. The rate is different for each nucleus, but constant for that nucleus. (All sorts of attempts have been made in laboratories to change the rate…with one oddball exception, absolutely nothing happened.) It’s a proportional thing; over some period of time, half of the atoms of some radioactive isotope will decay. You’re then left with a sample half the size of your original sample…and half of that will be gone after you wait the same period of time again. And so on. This period of time is known as the half life, because half of the atoms are gone after that period of time.
Of the things I’ve touched on, here are their half lives: Uranium-235: 703.8 million years. Potassium-40: 1251 million years. Uranium-238: 4458 million years. And Thorium-232: 14,050 million years.
And now maybe you can see how this might be useful to geologists. Find a rock with some uranium, thorium, or potassium in it. (Potassium most likely; it’s common compared to the others.) Then determine how much “daughter” product is in the rock. It helps if the daughter product is such it wouldn’t have been in the rock when it solidified. E.g., a zircon crystal, which might pick up uranium impurities as it crystalizes, but will positively reject lead atoms. Any lead in the zircon crystal can only have come from uranium decay. Count atoms (yes, you might have to literally count atoms) to determine how much daughter product there is, versus parent isotope. Figure out how much decay has taken place and compare to the half life.
You now know the age of the crystal. Not the relative age, the absolute age, of the crystal.
But there are a lot of details with this (including the fact that dating sedimentary rock is dicey), and I will cover some of them next time. These details, when fully considered only serve to make these methods rock solid.
Notice
I have a complex project coming up IRL, and I absolutely have to reallocate my “spare” time. This will mean less laughing at online flerfs, but it also means science posts will be infrequent and/or unpredictable.
