
The image of a “Suspicious Dog” is from Yours Truly’s files. The source is unknown, but to the best of Yours Truly’s knowledge and belief, it is not AI-generated.
There are Important Notifications from our host, Wolf Moon; the Rules of our late, good Wheatie; and, certain caveats from Yours Truly, of which readers should be aware. They are linked here. If readers wish to post anything that is AI-generated on the discussion thread for today’s special edition post, they must cite their source. Thank you.
Today’s STOP PRESS Edition post is devoted to one topic: the “Fast Track” approval that the FDA just granted to CSL / Arcturus Therapeutics for the company’s self-amplifying RNA (saRNA, aka sa-mRNA) Avian Influenza “vaccine”, ARCT-2304. Another version of this type of “vaccine” by the same company, called ARCT-154 or KOSTAIVE, for COVID-19 infection “prevention”, is already “fully approved” and in use in Japan and in the European Union / Scandinavia. Both of these “vaccines” are discussed in this article: https://finance.yahoo.com/news/arcturus-gets-fdas-fast-track-154900033.html, “Arcturus Gets FDA’s Fast Track Tag for Influenza Vaccine Candidate”, 11 April 2025. Two screenshots from this article are below:


Take note of the last sentence in the screenshot above: “A biologics license application for Kostaive in the United States is expected to be filed later in 2025.”
Now, the press release from Arcturus Therapeutics in January 2025, regarding ARCT-2304, found here: https://ir.arcturusrx.com/news-releases/news-release-details/acturus-therapeutics-announces-initiation-phase-1-h5n1-flu, “Arcturus Therapeutics Announces Initiation of Phase 1 H5N1 Flu Vaccine Trial”, 10 January 2025. The clinical trial for ARCT-2304 is registered here: https://clinicaltrials.gov/study/NCT06602531. Note that this is a PHASE 1 human clinical trial. Some of the details are below:
NCT06602531: Study Start Date: 12-12-2024
Primary Completion Date: 7-21-2025 (This means ONLY SEVEN MONTHS of a PHASE 1 human clinical trial before the Primary Completion Date. This is also a RED FLAG indication that Arcturus Therapeutics may likely apply for either a EUA or for a BLA (Biologics License Application, otherwise known as “full FDA approval”) BY THE LATE SUMMER / EARLY FALL OF 2025. In Yours Truly’s opinion, it is SCIENTIFICALLY IMPOSSIBLE to know in FEWER THAN AT LEAST TWO YEARS OF TESTING, WHETHER OR NOT A “VACCINE” ACTUALLY WORKS, LET ALONE BE “SAFE AND EFFECTIVE.”) In Yours Truly’s opinion, any claim by a “vaccine” manufacturer that a DNA-viral vector-based /RNA-based / “protein subunit”-based / “cell-based” / mRNA-based / saRNA-based / sa-mRNA-based “vaccine” — can be SAFELY developed, “tested” and granted either an EUA or “full approval” by the FDA, in fewer than at least two years, must be considered to be not only suspect — but as outright fabrication or wishful thinking.
Estimated Study Completion Date: 12-19-2025 (This would mean that the Phase 1 human clinical trial would be “completed” IN ONE YEAR PLUS ONE WEEK.)
Recruitment Information: Not Yet Recruiting
Contacts and Locations: NO locations are listed; there is only an 888 area code number to call the “Clinical Trials Disclosure Manager” for further information (which, by the way, said information would only be released to “researchers.” One assumes this means “degree-holding scientific researchers”, not to non-scientific-degree-holding researchers, let alone to the general public.)
Clicking on the Researcher View tab on the main study registration page yields some ** interesting ** information. Some examples: There will be a total of 200 persons used in the clinical trial; the ages will range from 18 years old to 80 years old; there is a “control group” that will receive injections of what appears to be a “standard influenza vaccine” plus, and/or, a saline placebo; and, there will be THREE levels of injectable used on the study subjects, at a “low” dose, a “medium” dose, and a “high” dose — of which, NO amounts of “vaccine candidate” are delineated; among other information.
A short summary of how saRNA (aka sa-mRNA) “vaccines” work is here: https://www.promegaconnections.com/how-do-self-amplifying-rna-vaccines-work/, by Jordan Nutting, 6 February 2024. Please see the screenshots from this article, below:
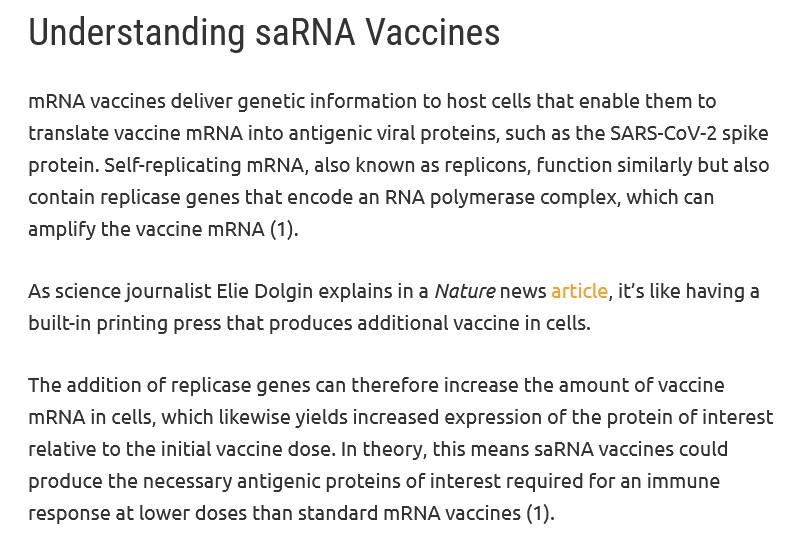
Note the language above regarding what saRNA does in the body: “...it’s like having a built-in printing press that produces additional vaccine in cells.” (Yours Truly: This “printing press” is at work in the body of the person who takes an saRNA “vaccine” for an unknown time — perhaps indefinitely.)


Note the language about the very long length of the mRNA sequences that must be used in saRNA (aka sa-mRNA) “vaccines.”
WHY IS THERE THIS UNHOLY RUSH TO GET ARCT-2304 THROUGH THE CLINICAL TRIAL PROCESS AND INTO EITHER EUA OR BLA STATUS WITH THE FDA, AND THEREFORE GET INTO USE IN THE UNITED STATES? In Yours Truly’s opinion, the answer may involve: Peter Marks, MD, PhD.
When it relates to a new drug or biologic product (including vaccines and other injectables), BOTH the FDA’s CBER (Center for Biologics Evaluation and Research) AND CDER (Center for Drug Evaluation and Research) departments are involved. Peter Marks, MD, PhD, was the director of CBER from 1 January 2016 (this made him an Obama administration holdover at the FDA) until his resignation from CBER on 29 March 2025 (his resignation became effective on 5 April 2025.) It appears that unless Dr. Marks resigned, he was going to be fired by now-HHS Secretary Robert F. Kennedy, Jr. Please see the screenshots below from this article on the situation (https://www.thefocalpoints.com/p/breaking-peter-marks-issues-veiled, “BREAKING – Peter Marks Issues Veiled Threat to America About Man-Made Biological Threats”, by Nicolas Hulscher, MPH, 5 April 2025.) The first is from Dr. Marks’ resignation letter; the other is from the article by Mr. Hulscher:

The screenshot below is from the interview transcript with Dr. Marks on CNN on 4 April 2025:

During this CNN interview, Dr. Marks made the “oblique threat” above.
On 2 April 2025, the FDA chose Scott Steele, PhD, as the Acting Director of CBER. Dr. Steele has been a full-time CBER advisor in late 2022 (this makes him a “Biden administration” holdover; and, Dr. Steele started with the FDA in June 2020 as an advisor in that agency’s Office of Medical Policy Initiatives.) Please see: https://www.fiercepharma.com/pharma/fda-taps-scott-steele-lead-cber-acting-basis-after-marks-departure, 2 April 2025. On 10 April 2025, the FDA granted “Fast Track” process approval for ARCT-2304. Who chose Dr. Steele to be the Acting Director of CBER on 2 April 2025?
In Yours Truly’s opinion, it is inconceivable that Dr. Steele and his colleagues at the related department of CDER, Dr. Jacqueline Corrigan-Curay, MD, and Peter P. Stein, MD — do not know what saRNA (aka sa-mRNA) does and how dangerous it can be to the human body; and, do not know that a “vaccine” product needs at least two to as long as five years to be properly developed, tested, results analyzed, and applications submitted to the FDA for EUA or for “full approval” of the injectable.
In Yours Truly’s opinion, what may be going on at the FDA regarding ARCT-2304 is a combination of an “end-run” around what Secretary Robert F. Kennedy, Jr., is trying to do to bring the agency into account for what is it supposed to do — to work in the best interests of the public health of the American people; plus, what appears to be personal bias against Mr. Kennedy, Jr., himself; plus, what appears to be a “H3ll-bent mindset” in the FDA to force the use of self-amplifying RNA products on the American people without going through the proper (lengthy) processes of testing, analysis, and proof of “safety and efficacy.”
Yours Truly presents the situation and her opinions. Readers can do their own due diligence and make their own conclusions.
FLASH! UPDATES, MONDAY 15 APRIL 2025:
First, this: https://twitter.com/RenzTom/status/1910780397899964560
Then, these: https://www.vigilantfox.com/p/fda-fast-tracks-vaccine-nightmare, 14 April 2025. Please scroll down the page to find the interview with Attorney Tom Renz; also: https://sayerji.substack.com/p/the-self-amplifying-rna-vaccine-threat, “The Self-Amplifying RNA Vaccine Threat and the Rise of BIo-Digital Warfare”, 11 April 2025. A screenshot from this article is below:

And, finally, from 2024: https://www.theqtree.com/2024/10/04/health-friday-open-thread-10-04-2024-self-amplifying-fda-sarna-a-primer-on-how-to-amplify-a-disaster/.
Peace, Good Energy, Respect: PAVACA