SPECIAL SECTION: Message For Our “Friends” In The Middle Kingdom
I normally save this for near the end, but…basically…up your shit-kicking barbarian asses. Yes, barbarian! It took a bunch of sailors in Western Asia to invent a real alphabet instead of badly drawn cartoons to write with. So much for your “civilization.”
Yeah, the WORLD noticed you had to borrow the Latin alphabet to make Pinyin. Like with every other idea you had to steal from us “Foreign Devils” since you rammed your heads up your asses five centuries ago, you sure managed to bastardize it badly in the process.
Have you stopped eating bats yet? Are you shit-kickers still sleeping with farm animals?
Or maybe even just had the slightest inkling of treating lives as something you don’t just casually dispose of?
中国是个混蛋 !!!
Zhōngguò shì gè hùndàn !!!
China is asshoe !!!
And here’s my response to barbarian “asshoes” like you:
OK, with that rant out of my system…
False Flag?
I think in some cases people on our side misuse False Flag. Unless, of course “FF” stands for something else.
This became apparent to me when I had a very valuable conversation with DePat and FG&C about the notion that the Arizona Audit people were waiting for a “FF” before dropping their results. Once FG&C explained what he meant by FF, it made a LOT more sense than it did with my reading of the term.
I first heard the term False Flag many, many years ago in an intelligence context. It’s a method of recruiting spies. The signature example is the KGB “handler” who finds someone in his host country who has access to classified information and is sympathetic to Israel, then arranges to meet the Israel sympathizer “by chance.” Once he does so he lets slip that he is an agent…but not for the USSR, rather for the Mossad. He’ll even explain that he knows government employees aren’t supposed to leak sensitive stuff but if the sympathizer could just alert him to harmless stuff, it’d help Israel out.
Before the Israel sympathizer knows it, he’s “helping Israel” a lot more than that, but in fact he’s really passing stuff on to the Soviet Union.
The thing that makes it “false flag” is that the Soviet agent, whose flag SHOULD be red with a yellow hammer and sickle in the upper left, is (figuratively) displaying a false flag–that of Israel.
In the more modern United States Cold Civil War context, a false flag is when some leftist does something while pretending to be on the Right, in the hopes that it will damage the Right politically. This is everything from posting a bunch of stereotypical “right wing hate” on the internet then going off and shooting up a black church (to prove “right wingers are racists”) to…well, January 6 with Antifa pretending to be “right wing militia” types–which was very damaging to us.
Just like the Soviet agent was pretending to be an Israeli agent, the leftist douchebag(s) is (are) pretending to be on the Right politically.
I can’t be certain but I suspect some conflate this with something different: A big spectacular event staged to distract from something they don’t want you to notice. False flags can certainly do this (have some “right wing nut” shoot up a school and that will indeed saturate the media for a few days) but not all such things are “false flags” because many of these events don’t try to discredit the Right.
Now the Opposition does pull that trick too, and quite often, but when they do so, it’s not a “false flag,” it’s something else with a name that may just be best described as “distraction” or “misdirection” (the magician’s term for such a tactic). Basically the staged event sucks all of the oxygen out of the media room and nothing else gets looked at for some short period of time (a day to a week). It doesn’t matter if it ends up making the Right look bad (though if it does, bonus!!!), if it keeps people from noticing something else that happened, the operation was a success.
In this particular instance, the suggestion was that the Audit Results We Have All Been Waiting For are being timed to drop when disgust with Biden reaches a (new) all time high. This is certainly plausible though I would have a multitude of detail questions about it before I’d go beyond that. But what this scenario does NOT describe is a “false flag.”
OK, that off my chest…lets hope that Arizona Audit drops soon. If that implies something else must happen first, then let THAT happen, already! Too much death and destruction is being meted out by the Biden Facade Administration and the people behind it.
Justice Must Be Done.
The prior election must be acknowledged as fraudulent, and steps must be taken to prosecute the fraudsters and restore integrity to the system.
Lawyer Appeasement Section
OK now for the fine print.
This is the WQTH Daily Thread. You know the drill. There’s no Poltical correctness, but civility is a requirement. There are Important Guidelines, here, with an addendum on 20191110.
We have a new board – called The U Tree – where people can take each other to the woodshed without fear of censorship or moderation.
And remember Wheatie’s Rules:
1. No food fights
2. No running with scissors.
3. If you bring snacks, bring enough for everyone.
4. Zeroth rule of gun safety: Don’t let the government get your guns.
5. Rule one of gun safety: The gun is always loaded.
5a. If you actually want the gun to be loaded, like because you’re checking out a bump in the night, then it’s empty.
6. Rule two of gun safety: Never point the gun at anything you’re not willing to destroy.
7. Rule three: Keep your finger off the trigger until ready to fire.
8. Rule the fourth: Be sure of your target and what is behind it.
(Hmm a few extras seem to have crept in.)
Spot Prices
All prices are Kitco Ask, 3PM MT Friday (at that time the markets close for the weekend).
Last week:
Gold $1817.80
Silver $24.08
Platinum $1016.00
Palladium $2498.00
Rhodium $18,400.00
This week, markets closed for the weekend at 3:00 PM Mountain Time
Gold $1828.60
Silver $24.77
Platinum $1032.00
Palladium $2506.00
Rhodium $17,750.00
Gold broke out and up into the 1830s this week but much of that gain was lost by close on Friday. Silver is up a bit too, the PGMs however are down (or steady).
I attended a talk about the silver market last week; the speaker actually alluded to the folks who pushed the price of the gaming company in order to try to bankrupt a bunch of institutional traders, and then went on to try the same with silver. He described their effort as a failure (and from what I’ve seen so far, their effect on silver prices was, in fact, minimal). However one effect that they did have was they got me to post articles on the nine precious metals AND give these updates every week.
Part XVII: Nuclear Physics Finds A Hammer
Introduction
Today, there is a subdiscipline of physics called “nuclear physics.” It deals with the nucleus of the atom, but does not typically dive any deeper than that (and there is most assuredly a “deeper than that” today known as “particle physics,” though there was no hint of its existence in the 1920s).
The sorts of investigations Rutherford and Co. performed in the first two decades of the 20th century were the very beginning of nuclear physics, though it’s often not considered to have been founded until 1932.
Why 1932? That’s the subject of today’s story.
There’s a modern trope among nuclear physicists. Someone asks “how do you find out what’s inside an atom” and the response is: “Just like a toddler trying to figure out what’s inside an alarm clock. He gets a hammer, smacks it, and sees what flies out of it.”
When we left off the physicist’s best subatomic hammer was the alpha particle, known to be a bare helium nucleus, mass number A = 4, electric charge +2. This would come flying out of certain atoms (like those of uranium and thorium) when they underwent what is called “alpha decay.” This process would reduce the atomic number (i.e., the element number, Z) of the parent nucleus by 2, and reduce its mass number, A, by 4. So uranium-238 (the isotope of uranium, Z=92, A=238) would become thorium-234; the mass number has decreased by four, and thorium is element #90, so the atomic number has dropped by 2.
Physicists used these alpha particles with some limited success as hammers to hurl at nuclei. In fact, that was how the nucleus had actually been discovered; Rutherford used alpha particles as a hammer on gold atoms and found there was a lot of empty space in an atom, but a very small hard kernel in the middle that would cause the alpha particles to ricochet. Physicists had even figured out how to give alpha particles more energy, by using electrically charged plates and so forth to get them to speed up.
But here’s the problem. The nucleus has a positive electrical charge, a substantial one. And an alpha particle, also a nucleus, has its positive electrical charge, too. And like charges repel each other.
Imagine if your hammer, and the nail you were trying to hit with it, strongly repelled each other. That’s a recipe for deciding a hammer is for hitting your thumb with, isn’t it? (Or perhaps your wrist, or even your face if the hammer bounces back at a sharp angle.)
Alpha particles were, to put it mildly, suboptimal as nuclear hammers.
There was also another glaring mystery in the early 1920s. What actually held a nucleus together?
As far as they knew back then, the nucleus of (say) oxygen-16 (Z=8, A=16) held a mixture of protons and electrons, 16 relatively heavy protons to give it the 16 mass number, and eight very light electrons (1/1836th the mass of a proton) to cancel out the charge of eight of the protons, leaving a net charge of 8, which was recently understood to be the very definition of an oxygen nucleus–a charge of eight.
It certainly looked as if there were electrons in a nucleus; consider beta decay. This is when the nucleus spits out an electron and goes up one in charge. For instance, the thorium-234 I referenced will spit out an electron (in this context, it’s known as a “beta particle”), uncovering another proton, raising the atomic number, therefore. from thorium’s Z=90 to Z=91, which means it’s now a protactinium-234 nucleus. So it certainly seemed as if nuclei had electrons in them; otherwise how on earth do electrons end up coming out of the nucleus during beta decay?
So let’s consider a helium-4 nucleus; under this model it contains four protons and two electrons. Those four protons can actually all touch each other (you can convince yourself of this with marbles, ping pong balls, or billiard balls). What keeps them from flying apart? The protons are all positively charged; and there are only two electrons to cancel that repulsion out.
Well, let’s list what we know about protons:
mass = 1.672×10−27 kg
electric charge, e = 1.602×10−19 C
radius = 0.8414 fm
[e is the symbol used for the electrical charge of a proton in particular; an electron has charge –e.]
[“fm” is “femtometer,” a femtometer is 10-15 meters, or a quadrillionth of a meter. Most people have heard the “nano” prefix, meaning one billionth; fewer have heard of pico (one trillionth), femto (one quadrillionth) or atto (one quintillionth).]
We can get an appreciation of the size of the problem by simply computing the electrical repulsive force between two protons that are touching each other. Their center-to-center distance is double the radius, or 1.6828×10-15 m, so we can plug everything into Coulomb’s Law to see how big the force is:
The vertical bars stand for “magnitude” (in other words, drop the vector stuff and just deal with the scalar values, because we want a size, not a direction.)
both Q values are the charge of the proton, e, and K = 8.988×109 Nm2/C2. You can do the math.
The answer I got is 81.456 newtons.
NOT 81.456 billionths of a newton, or trillionths of a newton, but 81.456 newtons. That’s the weight of 8.3 kilograms (81.456 N/(g=9.8 m/s2)) under Earth gravity.
This much force, between two itty, bitty, teensy, tiny particles!!! It’s an actual macroscopic amount of force. It’d be as if a proton could hit you so hard it’d be like taking a 60 mph pitch on the chin.
(Actually, now that you mention it: https://en.wikipedia.org/wiki/Oh-My-God_particle.)
The force is enormous compared to the size of the particles.
Since all four of the protons in the alpha particle touch each other, each proton is being repelled by three times this much force (244+ newtons). The two electrons that are attached to two of the protons attract with 167 newtons, but that still leaves 81 1/2 newtons of repulsion unbalanced, and that’s simply yuge.
Well, that’s the electromagnetic force. There’s one other force that could come into play: Gravity.
Now a physicist would know, instantly, that gravity doesn’t matter more than a mouse fart in a hurricane here, but many of you don’t, so let’s just check that.

The radius is the same, but the numbers of the masses are much lower than the numbers of the charges, roughly 1/100,000,000 as much. And G is only 6.67×10-11, much much less than K was, very roughly 1/100,000,000,000,000,000,000 as much.
I get 6.59 x 10-35 newtons.
“Drop in the bucket” doesn’t begin to describe that number in comparison to 81.456 newtons. Basically a quintillionth of a quintillionth the amount.
Nuclear physicists generally ignore gravity as a force between the objects they study. There’s no way its effect could even be measured as a fraction of the electromagnetic effect.
So, by everything known in the 1920s, nuclei should simply fly apart, in a nanojiffy. Or perhaps an attojiffy. The two fundamental forces act in opposite directions, but gravity shows up like Biden’s rally crowds showed up last year (and gravity can’t cheat to make up for that).
So by rights any nucleus bigger than hydrogen’s one-proton nucleus should simply fly apart. It should never have formed to begin with.
Since we’re still here, and not simply big Swalwellian clouds of hydrogen gas, clearly something else, something new, is at work.
And that is today’s story.
Can Nuclear Electrons Actually Exist?
Leaving aside the fact that the nuclear electrons can’t, all by themselves, keep a nucleus together, there was plenty of reason to question whether nuclear electrons even existed at all. There are, essentially, three reasons that I could explain to you. Number Three had to do with Dirac’s Equation which came along in 1928 and I want to save for another column. So going back to the other two reasons…
Issue #1: Binding Energy
In the introduction I described the prevailing model of the atomic nucleus as of the 1920s. Ernest Rutherford made the suggestion around 1919, but he decided shortly afterwards that it didn’t make sense; and this is one reason why.
One of the still-standing 1895 puzzles has to do with atomic weights. The atomic weight of, say, carbon is not quite twelve times that of hydrogen. Even after accounting for the presence of atoms with different mass numbers (uncommon isotopes of the same element), it still doesn’t quite work out; even accounting for all those nuclear electrons…it doesn’t work out.
In fact, heavier atoms (i.e., heavier than hydrogen) are always lighter than they would be if they were simple multiples of the proton’s mass, much less including some nuclear electrons as well. Even hydrogen-2 (deuterium) is less than twice the mass of hydrogen-1 (protium).
This, it turns out is due to something called binding energy. It’s the energy required to pull the protons apart.
This is directly analogous to the binding energy between, say, you and the earth. How much energy would it take to separate you from earth? At least as much as it would take to accelerate you to escape velocity. This is gravitational binding energy, because it’s the force of gravity that creates the potential difference between you standing on the surface of the earth, and you out in interstellar space.
It takes, very roughly, 7 million electron volts (MeV) to pull a proton out of a nucleus. Alternatively, if a proton is shoved into a nucleus, 7 MeV is released (just like, as you fall from a great height, you release a lot of kinetic energy).
That energy actually shows up on the books as missing mass. E = mc2, after all. So the particles in a large nucleus are all just a bit lighter in weight than they would be if they were separated; to separate them you have to add enough energy to make up the mass deficit.
If you were able to convert an entire proton to energy, it’d yield 938 MeV. The binding energy is therefore about seven tenths of one percent of the total mass/energy of the nucleus. We can actually measure that shortage…and, it turns out, had been measuring it for decades. This is the reason for the discrepant atomic masses.
Another sort of binding energy is the electromagnetic binding energy, keeping electrons in atoms. This ranges from a fraction of a single electron volt, to a bit over a dozen electron volts, for hydrogen. Is some fraction of the mass of an atom disappearing during chemical reactions, when chemical energy is released? The theory says yes. But it’s a small enough change (roughly one millionth the size of the nuclear binding energy) we haven’t actually measured it…yet.
I tried to discover exactly when this was first explained. It was sometime before the 1920s. Wikipedia says Einstein did it in 1905, but it simply points to the fact that he derived E=mc2 that year; I can’t quite nail down that he said, in that paper, that this is why nuclei heavier than protium are all lighter than they “should” be. If he did say that then, then I should have crossed off yet another mystery the week I talked about the incredible year Einstein had in 1905. If someone else (or Einstein himself) put two and two together after the fact…well, it certainly happened by the 1920s.
The reason I bring this up right now, is that it ties to the first issue with nuclear electrons. Ny Heisenberg’s uncertainty principle, an electron bouncing around in something as tiny as a nucleus must have a kinetic energy of at least 40 MeV (its position is very well defined, its momentum therefore isn’t going to be anywhere close to zero). Not only is this a lot more than the energy of beta radiation (presumed to be one of these electrons escaping the nucleus), it’s more than the binding energy of the protons; one bound electron bouncing around in there contains enough energy to kick five or six protons out of a nucleus! And what would keep it from flying out as super-energetic beta radiation?
Issue #2: Spin
Probing into quantum mechanics eventually established that protons and electrons have a spin of 1/2. Or, alternatively, -1/2.
But the term “spin” is misleading. The particles don’t actually spin like a top. They do something else that’s pretty whacky and has no sensible referent in day to day life. Nuclear and particle physicists will hijack an everyday term to describe these phenomena, however, so they speak of “spin.” They picked this word because it is measured in the same units as angular momentum. The actual value is 1/2 of ℏ, so the physicists simply label it “1/2.” It can point in two opposite directions, so the “other” direction is labeled -1/2.
If you have some even number of electrons or protons, they could be any combination of 1/2 and -1/2 spins, but since there is an even number of them, you can pair particles with 1/2 spin with particles of -1/2 spin, cancelling each other out, and some even number of particles will be an excess of 1/2 spin (or -1/2) spin particles. The excess will always be an integer, if there is no excess the total spin is zero–which is also an integer. (In practice, the + and – 1/2 spins will cancel each other as much as possible, in this case leaving a total spin of zero.)
An odd number, n of electrons or protons will always have 1/2 or -1/2 spin left over, on top of the integer spin that the even number n-1 of the particles will give.
So let us consider the nitrogen-14 nucleus (Z=7, A=14). It should have 14 protons and 7 electrons in it, which total to 21. Thus if the spin is measured, the net spin should have a 1/2 (or -1/2) fraction in it.
They did measure the spin of nitrogen-14 nuclei, and it always came out to integer spins. So there have to be an even number of protons plus electrons in that nucleus.
Therein lies an apparent contradiction, and there are no actual contradictions in reality; there must be some unknown fact or bad assumption that when identified, will resolve the apparent contradiction.
The Nuclear Force
I’ve described two issues with the concept of nuclear electrons. But I kind of skated past something in my discussion of binding energy. As I said, you are bound to the earth by gravity. Electrons are bound to atoms by the electromagnetic force. Protons are bound to a nucleus by…anyone? Anyone?
Clearly there’s some other force out there. A force strong enough to overpower the eighty newtons of force between adjacent protons. But weak enough that we’d otherwise never have noticed it–because we hadn’t noticed it. It should have been about as conspicuous as AOC in front of a TV camera, yet we never noticed it.
It seems odd to postulate a force that’s very strong at close quarters, yet unnoticeable at a distance. If were anything like electromagnetism or gravity, it should drop off as the square of the distance…twice as far away, you feel 1/4th the force, three times as far away, you feel 1/9th of the force. So if this hypothetical force is an attractive force stronger than the electromagnetic repulsion at some distance, it ought to still be stronger than the electromagnetic force twice as far away–both forces are a quarter as strong at that location as they were before, so the one that was larger before, should still be larger here.
But we all know of something that doesn’t behave that way, and that is magnets. Sure, one pole of a magnet has a force that drops off as the square of the distance, but there’s always a nearby opposite pole. If you’re right up against a north pole, the south pole of that magnet is, say ten times further away, and only cancels out 1/100th of the force. But double your distance from the north pole, and now the south pole is about five times further away and cancels out 1/25th of the force, as you move further and further away the two poles are (propotionally) closer to being the same distance away from you and cancel each other out quickly.
So magnetic forces appear to drop off as the cube of the distance from the magnet.
In order to match what we see, this hypothetical force should be almost nothing at 2.5 femtometers’ distance, strongly attractive at about 1 femtometer, and actually be repulsive at distances less than 0.7 femtometers. In other words, two protons would have to be almost touching for this force to become a factor.
The repulsion at very close distances actually puts a lower bound on the size of nuclei, since the protons can’t get closer than that without being pushed apart. That’s the effective size of a proton. And indeed these distances are roughly the size of a proton.
This force turns out to be very, very complex computationally, but it was consistent with everything they saw at the time, so, just like gravitational and electromagnetic forces, it was accepted as being true even if a lot of details needed to be ironed out. (And even though we know a lot more about it today (1920s physicists had no idea), there are still issues.)
Enter: the Neutron
I mentioned that even though Rutherford had originally suggested the nuclear electron, he grew dissatisfied with it for many of the reasons already mentioned, and a year later, in 1920, had come up with another idea. Perhaps, instead of proton/electron pairs, the extra, dead-weight mass of a nucleus that doesn’t contribute to its electrical charge was due to a neutral single particle about the mass of a proton. He even gave it a name, the neutron. This rather neatly solved the spin issue: If a nitrogen-14 nucleus contained 7 protons and 7 neutrons, the spins would add to zero. Repulsive forces would still be about the same, though: too much without positing a “nuclear force.”
But most physicists didn’t accept this conjecture. Though it solved a lot of the issues that the nuclear electron hypothesis introduced, physicists weren’t going to accept that this “neutron” thingie existed until someone actually detected one. Throughout the entire decade of the 1920s, most physicists continued to accept the nuclear electron hypothesis as being likeliest to be true, despite all the problems it seemed to raise.
If it seemed like this attitude was inconsistent with their fairly ready acceptance of the nuclear force, well…no. A force is intangible, but you can see its effects. You write some equations to build a model of how the force works, and if all of the effects match, you’ve probably got a good description of a real force, at least until you learn more. But if you posit a particle, you’ve posited something tangible that you should be able to detect in a much more direct way. And so far, the neutron had not been.
So we need to detect a neutron. But how? Protons and electrons are easy to detect, and relatively easy to manipulate, because they had electrical charges. One could see the effect of the electrostatic force, both caused by the particles, and also the effect of the force on the particles…in particular being able to deflect them to measure their mass, but also to accelerate them, like happened to electrons in a Crookes tube.
A totally neutral particle would be invisible based on these methods of detection…and impervious to being manipulated by electromagnetism.
But the first crack in this problem appeared in 1930. Walter Bothe and Herbert Becker, in Giessen, Germany, were using alpha particles from polonium (Z=94) in an experiment. They picked polonium because it spits out particularly energetic alpha particles (in other words, the alpha particles are moving faster than usual), and they wanted those energetic particles to use as a hammer on light elements, like beryllium (Z=4), boron (Z=5), and lithium (Z=3). When the alpha particles hit these light nuclei, an unusually penetrating radiation was produced. It couldn’t be deflected, so they tentatively concluded that these were very strong gamma rays. But it was hard to interpret the results definitively.Two years later, in Paris, Irene Joliot-Curie (the daughter of Marie and Pierre Curie) and her husband Frederic Joliot sicced this radiation on paraffin, a compound of carbon and hydrogen. It resulted in protons being ejected from the sample; the protons had kinetic energy of 5 MeV. This radiation, if it were gamma rays, would have to be 50MeV gamma rays, much stronger than anything seen to date.
Ettore Majorana, a young physicist in Rome, analyzed all this data and announced his conclusion: This radiation had to consist of neutral particles.
When Rutherford, and his Cavendish laboratories colleague James Chadwick had heard about the Paris experiments and they, too didn’t believe this radiation was any kind of gamma ray. Chadwick devised a bunch of experiments to prove it wasn’t gamma radiation, then went on to subject more materials to the mystery rays, and eventually demonstrated that whatever it was, it consisted of neutral particles about the mass of a proton.
In other words, Chadwick had found Rutherford’s neutron.
Now that the neutron had been found…whoosh!!! the nuclear electron hypothesis was discarded; the notion that a nucleus contained protons and (except for hydrogen-1) neutrons now made a lot of sense and we could be sure that neutrons actually existed rather than being a convenient shorthand.
Back to Binding Energy and the Nuclear Force
With the correct understanding of a nucleus consisting of protons and neutrons, things become a bit clearer. In many ways these particles are a lot alike, and collectively, they’re called nucleons. They are of almost identical mass, and both are subject to the nuclear force.
The mass number (A) of an isotope is now understood to be how many nucleons it contains. Atomic number (Z) is now strictly equal to the number of protons in the nucleus, since we no longer have additional protons masked by nuclear electrons. We now have a new number N, the number of neutrons, and N + Z = A.
Nucleons are bound together by the nuclear force, which is very short range, its maximum strength basically covers the distance from one nucleon to the next.
So picture a nucleus with (say) about sixty nucleons in it. A nucleon near the center of the nucleus is completely surrouned by other nucleons and they each exert a strong attractive force on it; the forces balance, that nucleon is pretty happy where it is. But note, this nucleon does not feel any attraction from a nucleon that is two nucleons away, rather than adjacent.
Nucleons near the surface of the nucleus only experience about half as much nuclear force, because they’re not surrounded by nucleons, they just see a few to one side of them…and again, no effect from the nucleons further away.
A very small nucleus, say carbon-12, has a large percentage of its nucleons at the surface of the nucleus, maybe a handful in the center are surrounded by other nucleons. This means that the average nuclear force on a nucleon is less than it is in larger nuclei, where most of the nucleons are surrounded by other nucleons.
Now, going to a very large nucleus, like that of uranium-238, the vast majority of nucleons are surrounded and thus tightly bound. But those near the surface, just like those on the surface of carbon-12, feel half of the nuclear force attraction. But the protons there actually feel more electrical repulsion, because that force is long range and there are a lot of other protons in that nucleus, all pushing them away. So that particular nucleus is teetering on the edge of falling apart. Indeed, given a few billion years, it will fall apart.
This is sort of a hand-wavy argument that the most stable nuclei are the medium size ones; ones where a large number of nucleons are completely surrounded (maximizing the attractive force they feel) but also where ones near the surface don’t get repelled by so many distant nucleons. Either side of that happy middle ground, the average nucleon either just feels less attractive force (smaller nuclei, fewer near neighbors on average to attract), or feels more repulsive electromagnetic force (larger nuclei, lots of protons repelling the nucleon).
The total nuclear binding energy of a nucleus can be plotted versus the number of nucleons; when you do this you get a diagonal line, down to the lower left, up to the upper right. It’s almost a straight line, but if you look closely, there’s a slight bend to it. (I’d show you but I can’t find that plot on line…and it’s not nearly as illuminating as the one I’m about to describe.)
If you then go through and plot the average binding energy per nucleon, you now get a very striking curve, like this:
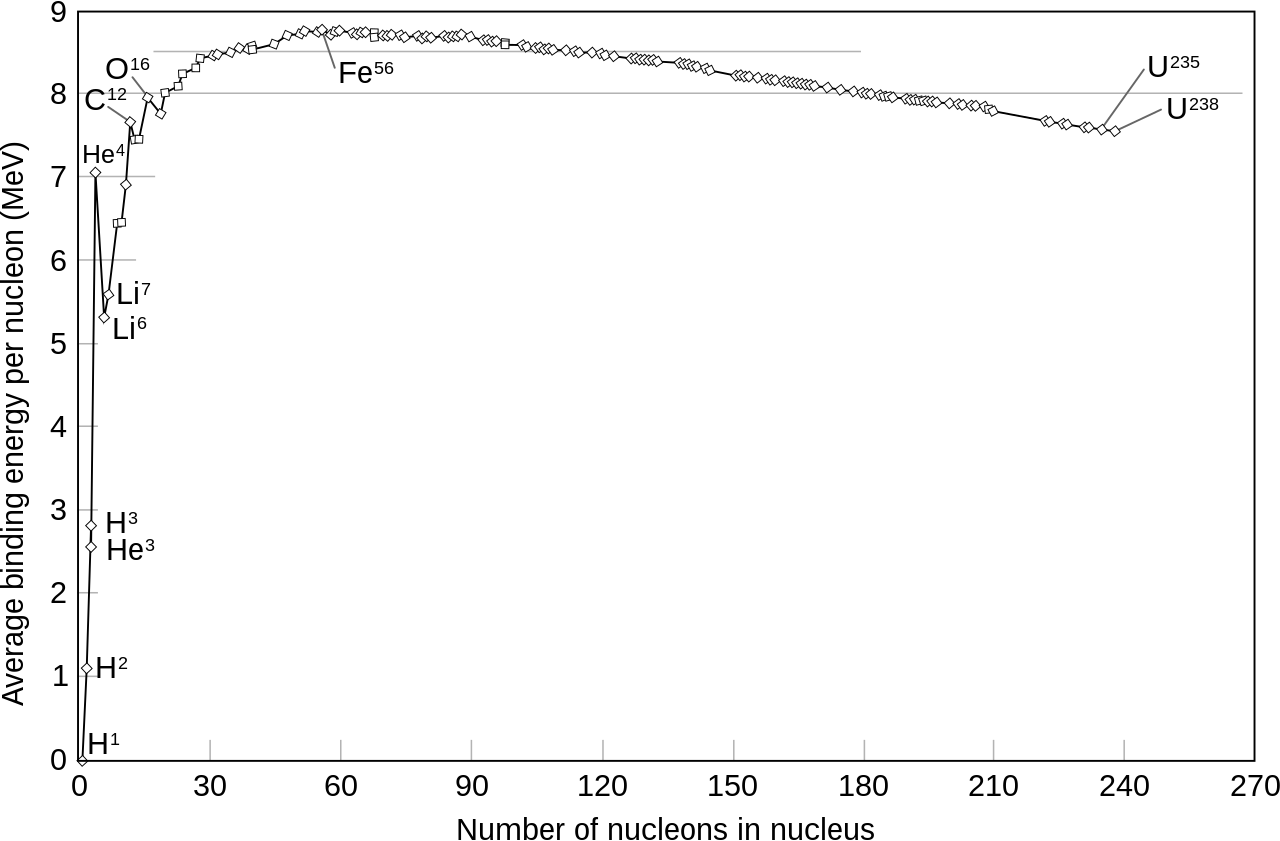
Now you can see that at about 56 nucleons, the binding energy per nucleon is highest; it takes more to pull one of those nuclei apart than any other nucleus. There’s a huge jump from hydrogen-1 (zero binding energy) to helium-4 (alpha particle).
Conversely, if you can build up to iron-56, you can release about 8 1/2 MeV per nucleon, which is a huge amount of energy. You can get most of that just going from hydrogen to helium-4.
Alternatively, if you can pull nucleons away from uranium-238, you can release about 1 MeV for each nucleon by the time you bring it down to iron-56. Uranium will actually help you get started on this by undergoing five alpha decays spontaneously as it decays to lead.
This was to have explosive implications. Quite literally.
But in the meantime, in 1920 Arthur Eddington–the same astronomer/physicist/mathematician who had measured the sun’s bending of the light from distant stars to prove general relativity correct just the year before–put forward the suggestion that perhaps this is what powered the stars…specifically the fusion of hydrogen into helium-4. In 1928 George Gamow did a lot of the math to figure out just what it would take to get this to happen. But hydrogen wasn’t thought to be any more common on stars than it is on earth. (The earth as a whole has little hydrogen in it; we think it’s common because there’s a lot of water up here on the surface). Cecilia Payne-Gaposhkin had, in her doctoral thesis in 1925, proposed that the sun was mostly hydrogen, but this was largely ignored because the prevailing theory was that the sun’s composition was similar to that of the earth. Eventually she was proved right, and Eddington, too was proved right. Most of the energy of stars does indeed come from hydrogen fusion; the rest comes from fusion of helium and heavier nuclei, releasing 7 MeV per nucleon. Further fusion happens in heavier stars to get that last 1 1/2 MeV / nucleon out of the “stuff” stars are made of. I discuss this in my older articles on stars, and we’ll be coming back to this in a future installation of this series.
[Semi-personal note: Gamow spent the last part of his career, 1956-1968, at the University of Colorado in Boulder (a/k/a “Berkeley by the Mountains”). This tower (physics faculty offices, one of the two or three tallest structures on the main campus with eight floors)…

…is named after him. (The physics lecture halls and labs are in the building at the bottom, and it looks like the picture was taken from a similar looking tower within which a lot of work is done for NASA–perhaps including the New Horizons probe that visited Pluto. I would cut through these buildings often going from one end of the campus to the other, particularly in bad weather. Football stadium in the background.)
The Neutron Hammer
Imagine that you are a lone proton, a/k/a an H+ ion, and you are headed directly towards, say, a carbon-12 nucleus. As you approach, you are slowed down by the repulsion of the six positively charged protons in that nucleus. If you aren’t moving very fast, you will eventually stop and be pushed away. If you are moving quite fast, you will get very close to that nucleus before stopping. If you are moving fast enough, you’ll manage to get close enough that suddenly, you’ll feel the nuclear force and now you’re caught–you just became part of a nitrogen-13 nucleus (which, by the way, is unstable and will want to decay–but not by either of the radioactive decay modes known so far).
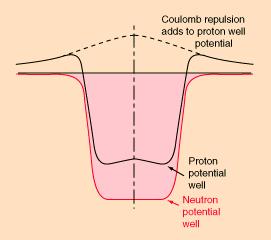
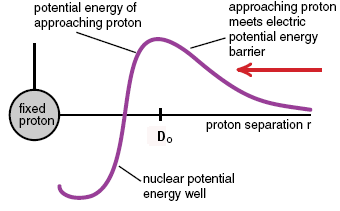 Imagine a proton coming in from the side, towards the nucleus (not shown) at center. It has to have enough velocity to travel over the “coulomb barrier” (repulsion from electrostatic forces), after which it can drop into the well because it is attracted by the nuclear force. This is actually a very good analogy because gravitational potential barriers are actual hills you’d have to be able to coast over. This one is a combination of the electrostatic and nuclear forces as they act on protons. In red is shown the situation for neutrons, which only respond to the nuclear force.
Imagine a proton coming in from the side, towards the nucleus (not shown) at center. It has to have enough velocity to travel over the “coulomb barrier” (repulsion from electrostatic forces), after which it can drop into the well because it is attracted by the nuclear force. This is actually a very good analogy because gravitational potential barriers are actual hills you’d have to be able to coast over. This one is a combination of the electrostatic and nuclear forces as they act on protons. In red is shown the situation for neutrons, which only respond to the nuclear force.Now imagine you are a neutron. You don’t feel any force at all, either repulsive or attractive, until just before impact, you feel the nuclear force, and now you’re caught like a fly on flypaper…you are now part of a carbon-13 nucleus (which is stable).
If you are a scientist looking to hit atomic nuclei with things, do you see that it might be fairly easy to hit nuclei with neutrons? Both protons and neutrons need to hit almost head on, but at least the neutron doesn’t need to be given a good hard shove just to get past the electrostatic repulsion.
Suddenly, it became very easy to take some perfectly ordinary, stable nucleus, like, for instance, calcium-42 (Z=20, A=42) and hit it with neutrons to make Ca-43, Ca-44 and so on. Eventually, you’ll get to a nucleus that’s unstable, Ca-45, which will beta decay to scandium-45 (Z=21, A=44).
There’s no calcium-45 found in nature on earth. It has to be made in a laboratory. But by irradiating various things with neutrons, isotopes like this, and literally thousands of others, were discovered, and their radioactivity studied. It turns out that every isotope that beta-decays releases a characteristic amount of energy when it beta decays, and usually the half lives are fairly short (days or years at most).
(Occasionally it turns out the half life is ridiculously long–quintillions of years, trillions of times the age of the universe, and it’s very hard to even tell that that isotope is radioactive. Only fairly recently, in fact, has it been proved that bismuth 209 (Z-83) is actually radioactive with a half life of 20 quintillion years; it had been considered a stable element, the heaviest one in fact, before then.)
In fact, you can turn this around. If you have a sample of unknown composition that has a lot of beta decay going on in it, you can measure the beta decay energy (or energies) and get a good idea what’s in the sample.
Which is well and good, but in most cases, your unknown sample will not consist of a bunch of these short-lived beta-decaying isotopes. They don’t exist in nature, unless they’re part of a uranium or thorium decay chain.
There’s a way around this. You can expose your sample to a strong beam of neutrons. Some of the atoms in it will capture the neutrons, become unstable isotopes, and reveal what they are. For instance, if you irradiate a sample with neutrons, and then detect Ca-45 decays, you know the sample must have a lot of Ca-44 in it (some of which captured neutrons and became Ca-45). Only a vanishingly tiny fraction of the atoms are altered by this treatment, but you do have the issue of your sample being radioactive for a while after the analysis is performed. This technique is effectively non-destructive since only a small fraction of the nuclei end up moving to the right one on the periodic table, and does see use, it’s called “Neutron Activation Analysis” (the neutrons are deemed to “activate” the nuclei by making them radioactive).
Neutron activation analysis will not tell you about what molecules a sample is made of, only what elements. So, for instance, if it detects some small amount of lead in a rock, you can’t know which ore of lead it is (though you might be able to infer it from what else is in the sample). An atom’s being in or out of a molecule has no effect on its radioactivity, which is what this analysis looks at.
Conclusion
The nuclear force is, today, considered the force that governs alpha decay, as well as nuclear fusion. As well as nuclear fission, but that had not been discovered yet. The neutron was going to be a very useful tool for nuclear physicists, and only thirteen years after it was discovered, the world would be slapped across the face with the realization that it had very practical applications as well.
We can cross a few 1894 mysteries off our list. But we have a new one to take their places.
If there are no electrons in the nucleus, what the heck is up with beta decay? Where does that zippy little beta particle, i.e., electron, come from?
Plus the mystery of the current age: Who the hell actually intentionally voted for Biden?
Obligatory PSAs and Reminders
China is Lower than Whale Shit
Remember Hong Kong!!!
中国是个混蛋 !!!
Zhōngguò shì gè hùndàn !!!
China is asshoe !!!
China is in the White House
Since Wednesday, January 20 at Noon EST, the bought-and-paid for His Fraudulency Joseph Biden has been in the White House. It’s as good as having China in the Oval Office.
Joe Biden is Asshoe
China is in the White House, because Joe Biden is in the White House, and Joe Biden is identically equal to China. China is Asshoe. Therefore, Joe Biden is Asshoe.
But of course the much more important thing to realize:
Joe Biden Didn’t Win
乔*拜登没赢 !!!
Qiáo Bài dēng méi yíng !!!
Joe Biden didn’t win !!!

