
The above is a vintage image of mass vaccination. (Courtesy Google Images.)
This series on the disaster of the COVID-19 virus itself, and of the COVID-19 “vaccines”, is dedicated to the memory of Yours Truly’s cousin Bill, who “died suddenly and unexpectedly” in September 2023.
The origination of today’s post begins here: www.dossier.today/p/double-digits-biden-admin-tells-americans, “Double Digits: Biden Admin tells Americans that it’s soon time for their 10th Covid shot“, by Jordan Schachtel, 13 June 2024. (Mr. Schachtel wrote about the ninth COVID-19 “vaccine” injection here: www.dossier.today/p/dose-number-nine-cdc-panel-green, “Dose number NINE: CDC panel green lights yet another Covid mRNA shot“, 29 February 2024. The CDC recommended that persons over age 65 take another “booster shot” of either the Pfizer-BioNTech or of the Moderna “2023-2024 Formula COVID-19 Vaccine” of these manufacturers.) A person age 65 or older, if that person adhered to every CDC recommendation regarding taking a COVID-19 “vaccine” injection since December 2020 (when the FDA granted first Emergency Use Authorization (EUA) to Pfizer-BioNTech and to Moderna for these companies’ “flagship” modRNA COVID-19 “vaccines” (BNT162b2 by Pfizer-BioNTech; and, mRNA-1273 by Moderna), would have taken injection number nine starting on 28 February 2024.
Today’s post is long. There is a large amount of information to “unpack.” Stay with me here.
Below is an image from the FDA’s 13 June “updated” authorization announcement for the “2024-2025 Formula COVID-19 Vaccine”, the TENTH injection dose of the modRNA “vaccine” formula: www.fda.gov/vaccines-blood-biologics/updated-covid-19-vaccines-use-united-states-beginning-fall-2024.
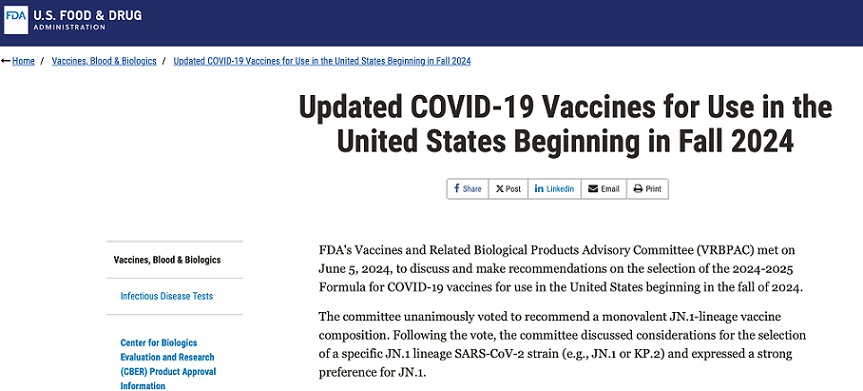
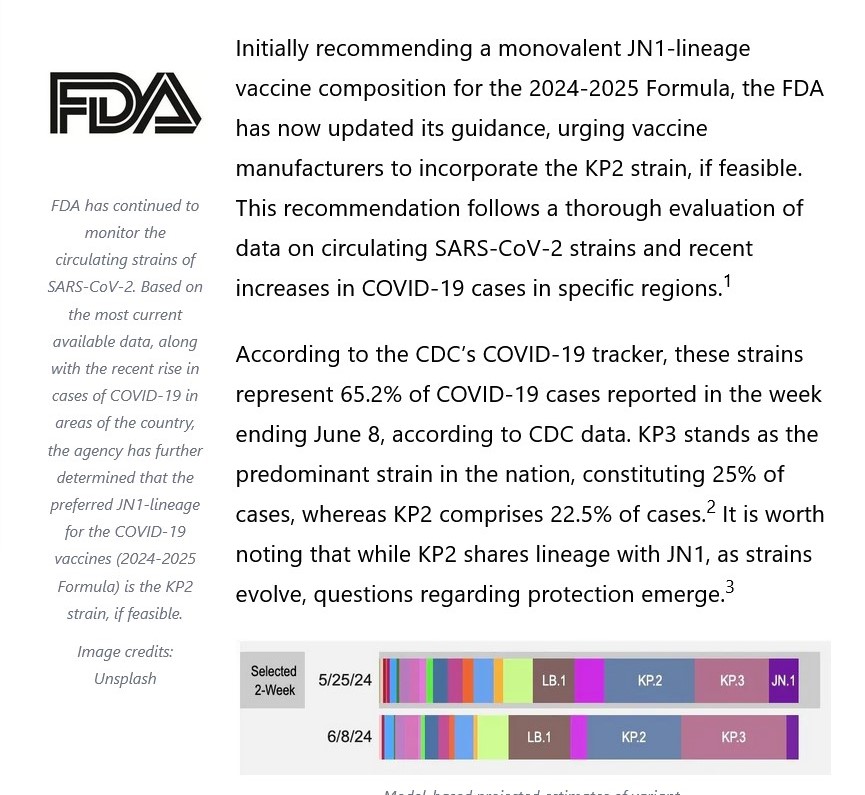
Note the language regarding the “selection of a specific JN.1 lineage SARS-CoV-2 strain…” More about that later.
The trail behind the 5 June 2024 FDA announcement begins with the VRBPAC Briefing Document for the meeting held on 28 June 2022: www.fda.gov/media/159452/download, “FDA Briefing Document Vaccines and Related Biological Products Advisory Committee Meeting June 28, 2022.” It was at this meeting that the FDA “codified” the types of “strain composition recommendations” that the agency would use regarding “new versions” of COVID-19 “vaccines.” Yours Truly presents page 17, page 18, and page 19 of this document:

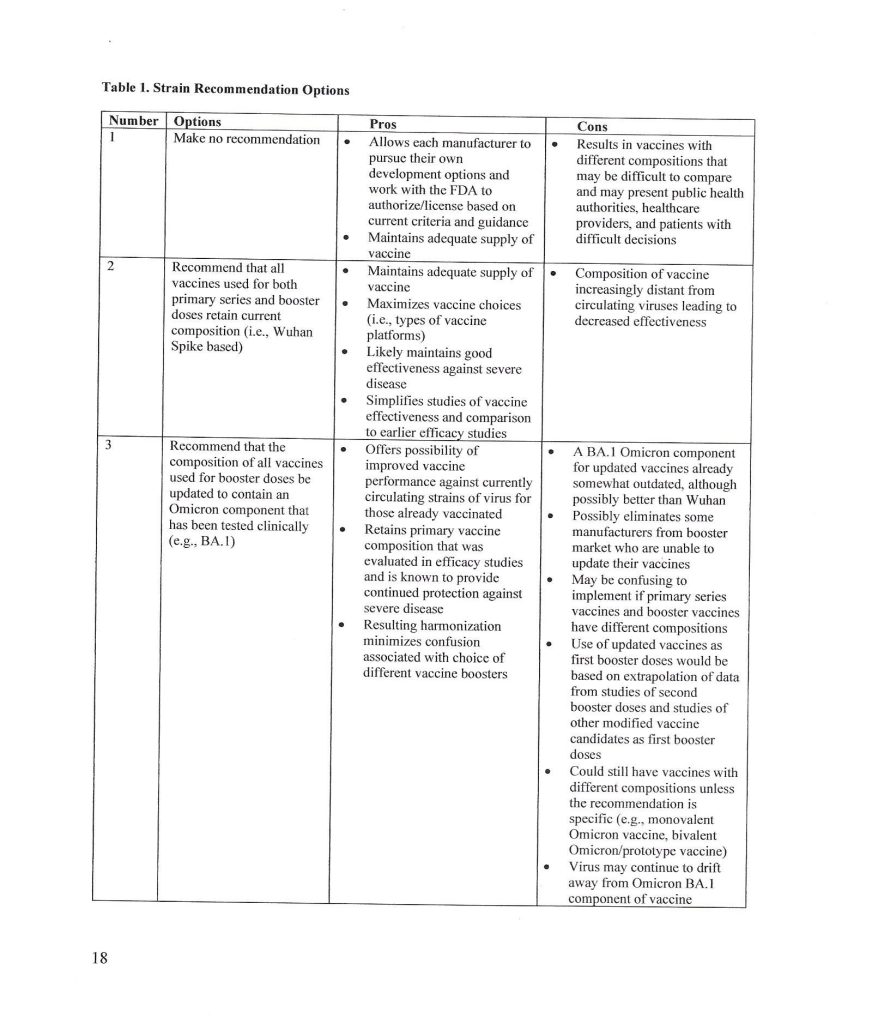
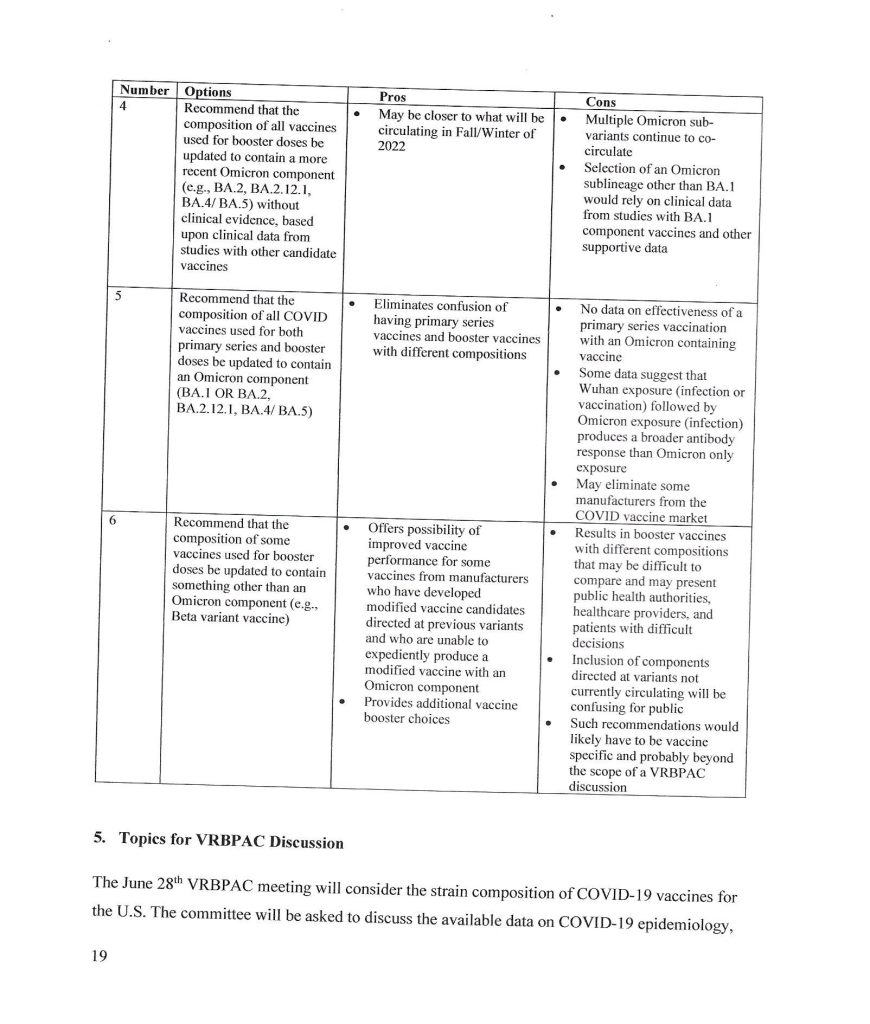
It appears that the FDA simply decided that it would be permissible for the agency to authorize a new COVID-19 “vaccine” strain composition along what, in Yours Truly’s opinion, may be called “very flexible” options. For example, the Pfizer-BioNTech XBB.1.5 COVID-19 “vaccine”, which was FDA authorized in the fall of 2023, had test results only from mouse testing prior to FDA authorization. Following are: The link to the Pfizer-BioNTech slide presentation about this “vaccine” to the CDC’s ACIP committee (Advisory Committee on Immunization Practices) meeting of 12 September 2023; and, an image of slide CC4 from this presentation. First, the presentation: www.cdc.gov/vaccines/acip/meetings/downloads/slides-2023-09-12/10-COVID-Modjarrad-508.pdf.
Second, slide CC-4 from the above presentation:
The XBB.1.5. Pfizer-BioNTech COVID-19 “vaccine” had only been given as a single injection to humans in the company’s clinical trial; a clinical trial which had only just begun prior to the ACIP meeting. Slide CC-5 of the presentation, the start of the company’s human trial of this “vaccine”, is below:
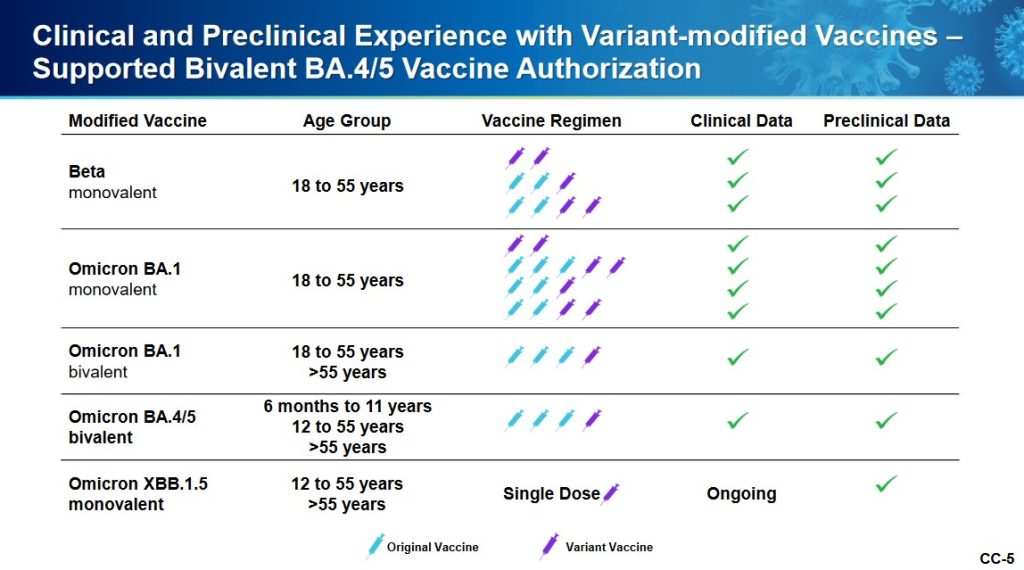
Slide CC-6 of the presentation has to do with the mouse studies of this “vaccine”, which were of longer duration.
Notwithstanding the above, the FDA authorized the use of the company’s XBB.1.5 COVID-19 “vaccine” on 11 September 2023 (in Yours Truly’s opinion, it appears that the ACIP meeting of 12 September 2023 was a “catch-up” formality.) It also appears (again, in Yours Truly’s opinion), that the FDA used a very loose interpretation of “Option 4” on page 18 of the FDA Briefing Document above in granting the EUA for this “vaccine”.
** Now, on to the latest “new version” of the COVID-19 “vaccines”, the “2024-2025 Formula COVID-19 Vaccines”, that the FDA authorized in June 2024.
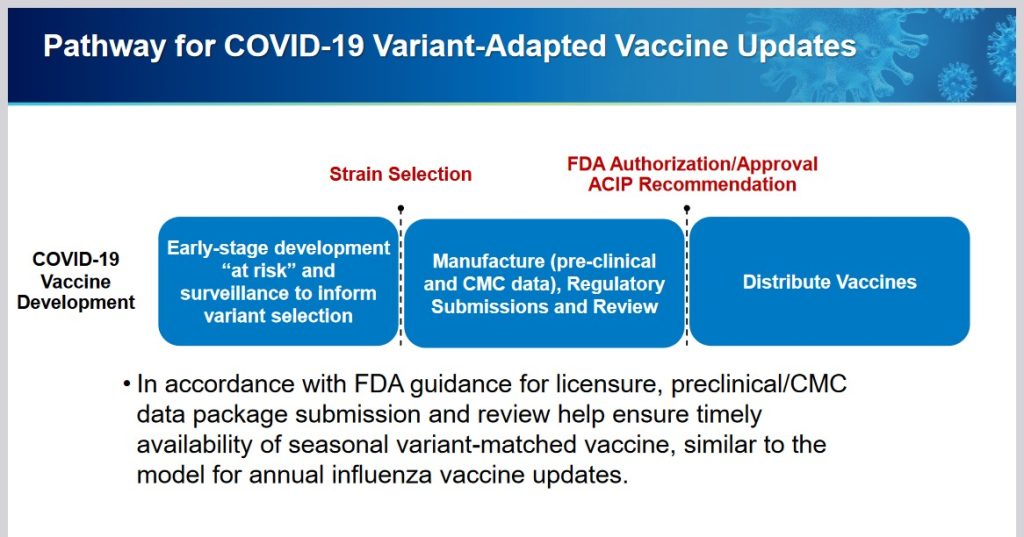
The following linked items are important regarding background information related to this situation and to the FDA: First, the FDA document, stating that the agency would “align” its COVID-19 “vaccine” antigen composition to the recommendations of the World Health Organization’s TAG-CO-VAC recommendations: www.fda.gov/media/179139/download (the TAG-CO-VAC recommendation for the “2024-2025 Formula COVID-19 Vaccines” was to use the JN.1 strain); second, the FDA document regarding “considerations and recommendations” for the “2024-2025 Formula COVID-19 Vaccine” composition: www.fda.gov/media/179145/download; third, the FDA announcement of the 5 June meeting of its VRBPAC committee (Vaccines and Related Biological Products Advisory Committee.): www.fda.gov/advisory-committees/advisory-committee-calendar/vaccines-and-related-biological-products-advisory-committee-june-5-2024-meeting-announcement. From this last link, chick on “Event Materials” to see the slide presentations and other items that were discussed at this meeting.
Two important items from the “Event Materials” list: the FDA Briefing Document; and the VRBPAC roster for this meeting. First, the FDA Briefing Document: www.fda.gov/media/179003/download; and, second, the VRBPAC roster for this meeting: www.fda.gov/media/179225/download. The roster for the 5 June 2024 meeting has some “familiar” members and speakers: Paul Offit, MD; and Peter Marks, MD (director of CBER [Center for Biologics Evaluation the Research of the FDA]); and, among the “Temporary Voting Members”, are: Bruce Gellin, M.D., M. PH., the Chief of Global Public Health Strategy for the Rockefeller Foundation; and, Melinda Wharton, M.D., M. PH., Associate Director of Vaccine Policy of the CDC. (Italics mine)
The VRBPAC members voted unanimously to endorse the Pfizer-BioNTech, the Moderna, and the Novavax “2024-2025 Formula COVID-19 Vaccine” by these companies, based on the presentations of these companies’ representatives at the meeting. Yours Truly can find no registered human clinical trials performed in advance of the 5 June VRBPAC meeting by Pfizer-BioNTech, or by Moderna, or by Novavax, for any “2024-2025 Formula COVID-19 Vaccine”; that would indicate that any “clinical trials” were performed in these companies’ facilities on mice; and that any “human trials” were also performed in these companies’ facilities, prior to the meeting. The FDA then issued the agency’s original announcement of 7 June 2024: www.fda.gov/news-events/press-announcements/fda-roundup-june-7-2024; and, a screenshot from this announcement:

Note in particular “…the selection of a specific JN.1 lineage SARS-CoV-2 strain (e.g., JN.1. or KP.2) and expressed a strong preference for JN.1.” Here’s where it starts to “get interesting.”
First, on 12 April 2024 (well ahead of the 5 June VRBPAC meeting), Pfizer-BioNTech issues a statement regarding the company’s “taking reservations” for the coming “2024-2025 Formula COVID-19 Vaccine” (also, see the Pfizer-BioNTech presentation at the 5 June meeting, linked above): www.cvdvaccine-us.com/reservation. This is followed, after the meeting, by Moderna filing an application with the FDA for a “vaccine” to target the JN.1. COVID-19 strain (also, see the Moderna presentation at the 5 June meeting, linked above): https://investors.modernatx.com/news/news-details/2024/Moderna-Files-FDA-Application-for-the-JN.1-Targeting-COVID-19-Vaccine/default.aspx; then, Novavax files with the FDA for that company’s version (also, see the Novavax presentation at the 5 June meeting, linked above): https://ir.novavax.com/press-releases/2024-06-14-Novavax-Submits-Application-to-U-S-FDA-for-Updated-Protein-based-2024-2025-Formula-COVID-19-Vaccine.
But then, “something happens”, and the FDA suddenly makes a large “about-face” and switches its “2024-2025 Formula COVID-19 Vaccine” choice to the KP.2 strain on 13 June 2024: www.fda.gov/vaccines-blood-biologics/updated-covid-19-vaccines-use-united-states-beginning-fall-2024. This is the “second” announcement, which was cited at the beginning of today’s post.
What was it that happened? Part of the answer lies in the fact that the NIH and Moderna co-own the patents (and, therefore, share the royalties) for the Moderna “flagship” modRNA COVID-19 “vaccine”, mRNA-1273. This agreement would extend to “descendant clone COVID-19 vaccines” by Moderna. www.citizen.org/article/modernas-mrna-1273-vaccine-patent-landscape/. The NIH’s Dale and Betty Bumpers Vaccine Research Center (part of NIAID — which Dr. Anthony Fauci led from November 1984 until his retirement in December 2022) and Moderna co-developed mRNA-1273. https://covid19.nih.gov/news-and-stories/nih-vaccine-research-center; a screenshot from the article is below:

The other part of the answer is that Moderna was already developing a KP.2 strain COVID-19 “vaccine” for 2024-2025. This, and the FDA’s decision to shift away from the JN.1 strain to the KP.2 strain, are described in this post at Sasha Latypova’s blog: https://sashalatypova.substack.com/p/all-roads-lead-to-resilience, “All Roads lead to Resilience. FDA is removing competitors for the Pentagon & CIA’s baby…Moderna”, 23 June 2024.
The FDA’s “about-face” announcement regarding the switch from the JN.1 strain to the KP.2 strain was also covered here: www.contagionlive.com/view/fda-advises-manufacturers-to-consider-kp-2-strain-for-covid-19-vaccines, 14 June 2024, by Sophia Abene. Below is a screenshot from this article:
However, there’s yet another detail in play here, regarding the FDA’s switch, “based on evaluation of the most recent circulating strains of COVID-19”, from JN.1 to KP.2 — the CIA and the Pentagon. Here is a screenshot from Sasha Latypova’s Substack article:

Here is the report, linked from the Latypova blog article cited above, that describes the CIA-linked company, “National Resilience”, or “Resilience”, that manufactures the RNA for the Moderna modRNA line of COVID-19 Omicron “vaccines”: https://unlimitedhangout.com/2022/08/investigative-reports/rna-for-modernas-omicron-booster-manufactured-by-cia-linked-company/, by Whitney Webb, 17 August 2022. Below is a screenshot image from this blog article:

And here is story on this “interesting development”, from Resilience: www.businesswire.com/news/home/20210908005443/en/Resilience-to-Manufacture-mRNA-for-Moderna’s-COVID-19-Vaccine, 8 September 2021. Note that per this “multi-year contract”, Resilience manufactures the mRNA for the Moderna COVID-19 “vaccines” at this Canadian facility. Resilience was founded in 2020.
But wait, there’s more! Resilience lists multiple “partners”, such as the Mayo Clinic. The company also, apparently, has a “partnership” with the United States Army’s Joint Program Executive Office for Chemical, Biological, Radiological and Nuclear Defense https://resilience.com/learn/partnerships. Below is a screenshot from this website:

The website link in the screenshot above is broken. Here is the Army’s website on this: wwwt2.army.mil/T2-Laboratories/Designated-Laboratories/Joint-Program-Executive-Office-for-Chemical-Biological-Radiological-and-Nuclear-Defense/. Note: this link may or may not work. One will need to do a search for “Joint Program Executive Office for Chemical, Biological, Radiological and Nuclear Defense” to see links to this department of the United States Army. One such link: https://globalbiodefense.com/directory/name/joint-program-executive-office-for-chemical-biological-defense-jpeo-cbd/.
It appears, then, in Yours Truly’s opinion, that the FDA was perhaps “reminded” of the”details” regarding the NIH-Moderna co-ownership (and royalties – sharing) agreement related to Moderna’s modRNA COVID-19 “vaccines”; and, the role of the CIA-Pentagon-National Resilience (aka Resilience) in manufacturing the mRNA used in the Moderna COVID-19 Omicron “booster vaccines” — and the KP.2. strain is indeed a “descendant strain” in the Omicron lineage (as is the JN.1 strain.) Hence, the FDA’s 2024-2025 COVID-19 “vaccine” strain “sudden switch” announcement of 13 June 2024, only one week after the agency gave the nod to the JN.1 strain.
In Yours Truly’s opinion, it is statistically, medically, and ethically impossible for a new vaccine (let alone any COVID-19 “vaccine”) to be developed; tested (on lab animals, then on human subjects); the test data thoroughly collated and analyzed for “safety and efficacy” on both lab animals and on human subjects; then, which data is presented to the CDC / FDA for consideration; then, these agencies doing their own “due diligence” research; then, and only then, being granted an EUA by the FDA; then, and only then, manufactured for use in humans — in a time span of fewer than three to five years, let alone within a time span of only a few months. It appears, again in Yours Truly’s opinion, that the CDC and the FDA are playing “fast and loose” with the health and safety of the people who choose (or will be “mandated”) to take the “2024-2025 Formula COVID-19 Vaccine.” And, also, that “other entities” are in play here to perhaps “influence” decision making by these agencies.
All of above is in addition to the fact that the COVID-19 “vaccines” (actually, gene therapy injections) have caused, are causing, and will cause, multiple health issues, serious adverse reactions, and deaths, in those who are “vaccinated.” Just two of the most recent discoveries: One, the COVID-19 “vaccines” can cause brain damage, an article by Dr. William Makis: www.globalresearch.ca/brain-damage-covid-19-mrna-vaccines/5861012, “Brain Damage Caused by COVID-19 mRNA Vaccines”, 26 June 2024. Below is a screenshot from Dr. Makis’ article:

The second most recent discovery, that the COVID-19 “vaccines” reduce life expectancy (even in “all-cause” analysis) among COVID-19 “vaccinated” persons, by Dr. Peter A. McCullough: https://petermcculloughmd.substack.com/p/breaking-publication-a-critical-analysis, “BREAKING Publication — A Critical Analysis of All-Cause Deaths during COVID-19 Vaccination in an Italian Province”, 1 July 2024. The peer-reviewed paper is here: https://doi.org/10.3390/microorganisms12071343, “A Critical Analysis of All-Cause Deaths during COVID-19 Vaccination in an Italian Province”, Marco Alessandria, et al., published 30 June 2024. Below is a screenshot from the Conclusions section of this paper:

In Yours Truly’ opinion, it is apparent at “half a glance” that the COVID-19 “vaccines” (actually, gene therapy injections) must be completely withdrawn for human use until these products have been fully investigated, and then re-designed, before being re-introduced for human use; and, that there is no “co-ownership” or sharing of royalties between a government agency and a COVID-19 “vaccine” manufacturer; and, that there is no involvement of the United States military in the development or manufacture of such products.
Peace, Good Energy, Respect: PAVACA






























