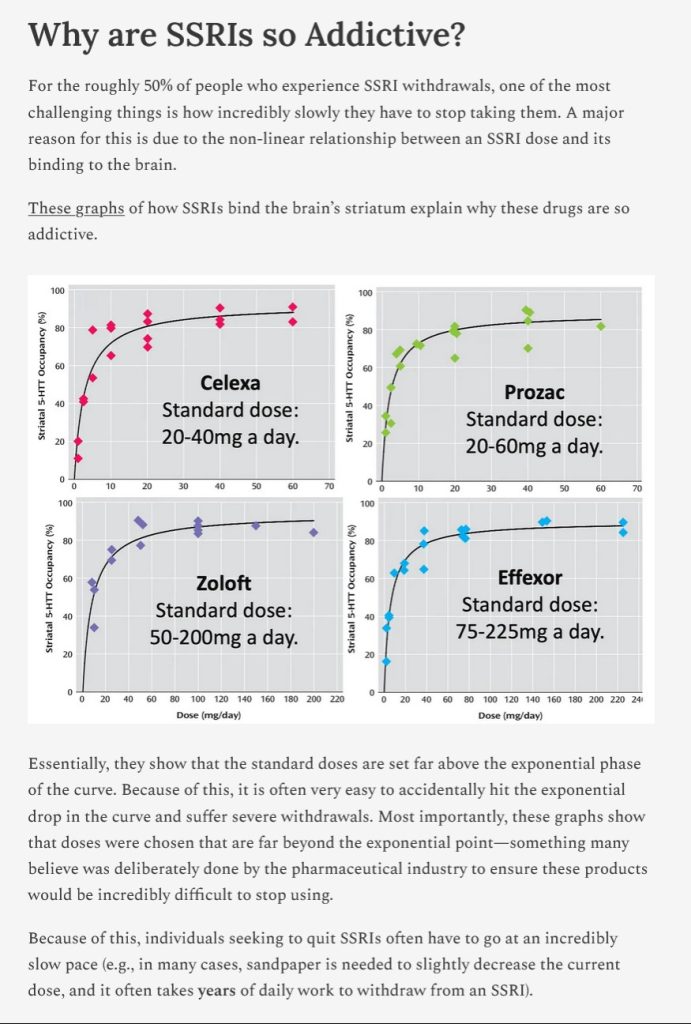
The free vintage header image of an architectural drawing is courtesy of iStock and Google Images.
Health Friday is a series devoted to information about Big Pharma, vaccines, general health, and associated topics. As today’s offering speaks to the disaster of COVID-19, Yours Truly dedicates it to all persons, of whatever age or location, who have suffered injuries, illnesses, or disabilities; or, who have passed away, from an infection of the COVID-19 BIOWEAPON virus itself, or from having been injected with any of the COVID-19 BIOWEAPON “vaccines.”
There are Important Notifications from our host, Wolf Moon; the Rules of our late, good Wheatie; and, certain caveats from Yours Truly, of which readers should be aware. They are linked here. Note: Yours Truly has checked today’s offering for AI-generated content. To the best of her knowledge and belief, there is none, except perhaps for AI-generated content within linked URLs. If readers wish to post AI-generated content in today’s discussion thread, they must cite their source. Thank you.
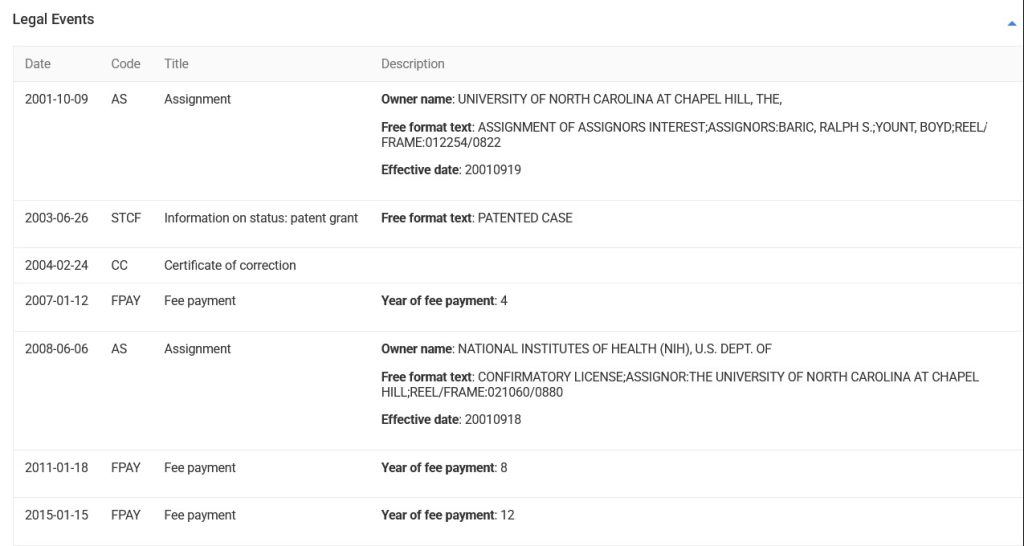
Special Note regarding today’s offering: In no way is the following, or The Baric Files series in general in Health Friday posts, to be construed as a “character assassination”, or as an “indictment”, or as any other type of attack or smear on Ralph S. Baric, PhD, of the UNC Gillings School of Global Public Health of the University of North Carolina, Chapel Hill, or of his lab at the university. The linked scientific papers, images therefrom, and other information about Dr. Baric (such as his Curriculum Vitae [1]) are all available on the internet. The ideas and conclusion of today’s offering are by Yours Truly. There is a plethora of items related to Dr. Baric; Yours Truly will focus on some of them in each part of The Baric Files.
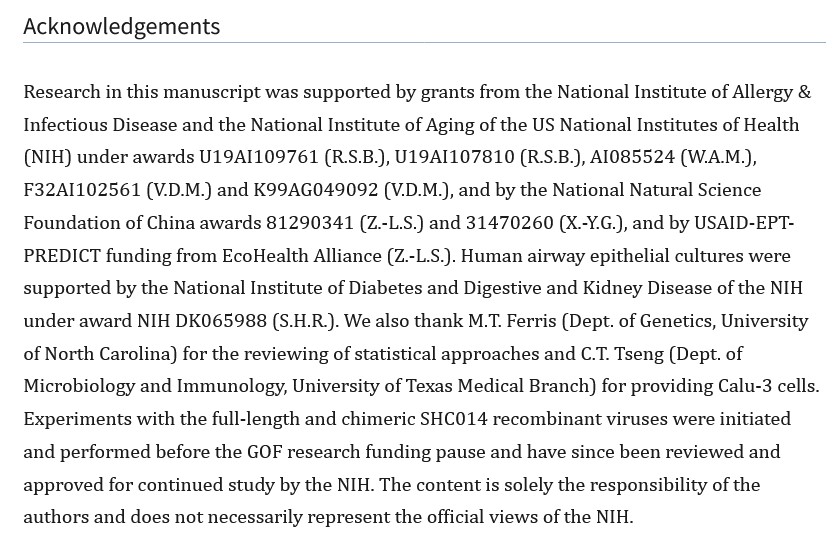
Yours Truly wishes to thank our host, Wolf Moon; Gail Combs; and others on the board here, who have brought information to light regarding the COVID-19 disaster (the BIOWEAPON virus itself; the BIOWEAPON “vaccines”; and the fallout from both), including information regarding Dr. Baric. I hope The Baric Files series has added to the knowledge base. In the course of producing The Baric Files, Yours Truly has read multiple scientific papers — blog articles — documents — grants information and applications — and, other pieces of information related to Dr. Ralph Baric, PhD. Even with all of this, however, it represents only a small part of the vast amount of information related to Dr. Baric and his research journey.
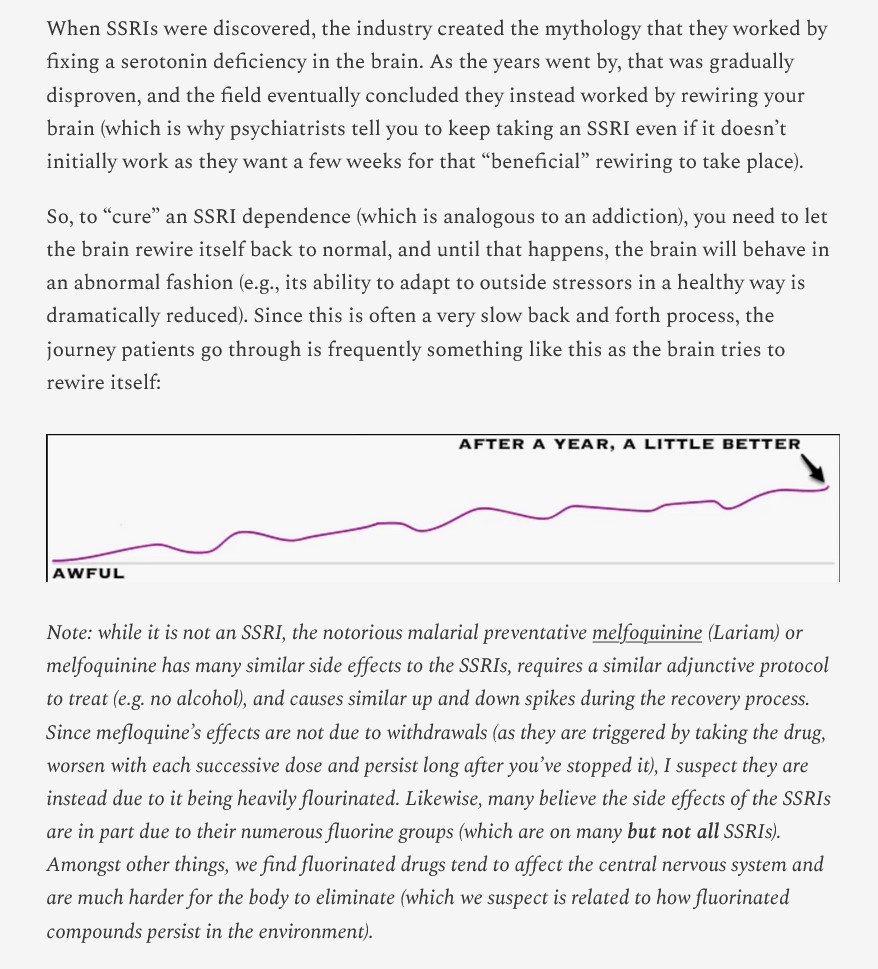
What Yours Truly has read has done several things for me: One, to understand more of how deep and wide was the scope of funding and research into the lab-creation of the SARS-CoV-2 (COVID-19) BIOWEAPON virus; Two, to understand more of how deep and wide was the scope of involvement of the United States military in this effort; Three, to understand more of the sheer magnitude of the damage potential that the lab-created SARS-CoV-2 BIOWEAPON virus had built into it; Four, to understand more of how this virus, which is the basis of ALL of the COVID-19 BIOWEAPON “vaccines”, had its damage potential “transferred” to these injectables; Five, to understand more of why this virus, and the “vaccines”, have forever changed humankind in multiple negative ways; and, Six, to understand more of why ALL of the COVID-19 BIOWEAPON “vaccines” MUST be immediately removed from the market and from use in the United States.
One more important thing to be understood, in Yours Truly’s opinion, by anyone who reads today’s offering, and indeed, The Baric Files series, is how one scientist — Dr. Ralph Baric, PhD — devoted over thirty years of focused, patient, methodical research into lab-creating the “template” for the modern scourge of humankind — the SARS-CoV-2 virus. In a way, it is like learning about how an artist — or, an architect — took years to create their masterpiece. Except, that in the case of Dr. Baric’s lab-creation of the SARS-CoV-2 virus “template”, it was a very different type of “masterpiece.” And, for this “masterpiece”, the creator / inventor was granted a US Patent.
The previous posts of The Baric Files are here: Part One: https://www.theqtree.com/2025/12/05/health-friday-open-thread-the-baric-files-part-one/; Part Two: https://www.theqtree.com/2025/12/12/health-friday-12-12-2025-open-thread-the-baric-files-part-two-of-mice-rabbits-and-ai-23946/; Part Three: https://www.theqtree.com/2026/01/02/health-friday-1-2-2026-open-thread-the-baric-files-part-three-patents-dr-anthony-fauci/; Part Four: https://www.theqtree.com/2026/01/09/health-friday-1-9-2026-open-thread-the-baric-files-part-four-ecohealth-alliance-wuhan-institute-of-virology-dr-zheng-li-shi-the-template-patent/; Part Five: https://www.theqtree.com/2026/01/16/health-friday-1-16-2026-open-thread-the-baric-files-part-five-united-states-department-of-defense-now-department-of-war-18-u-s-c-2339-18-u-s-c-1001-and-15-u-s-c-1-3/.
Today’s offering is Part Six of six.
Recent NIH Grants to Dr. Baric:
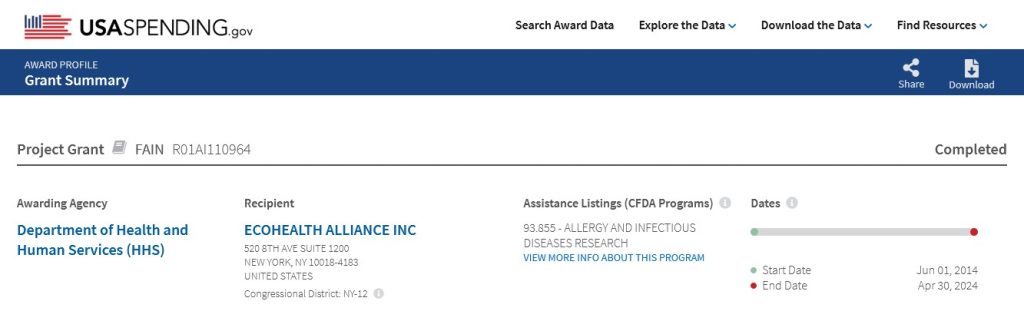
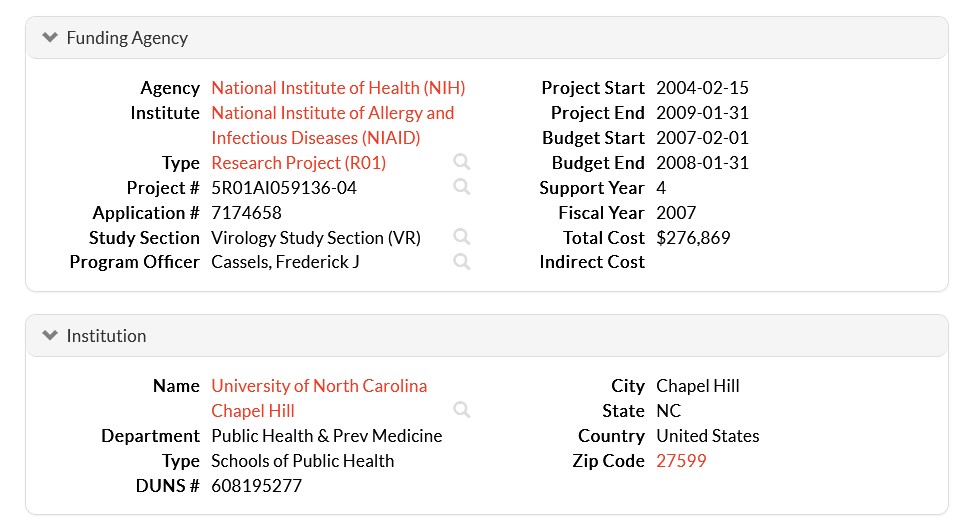
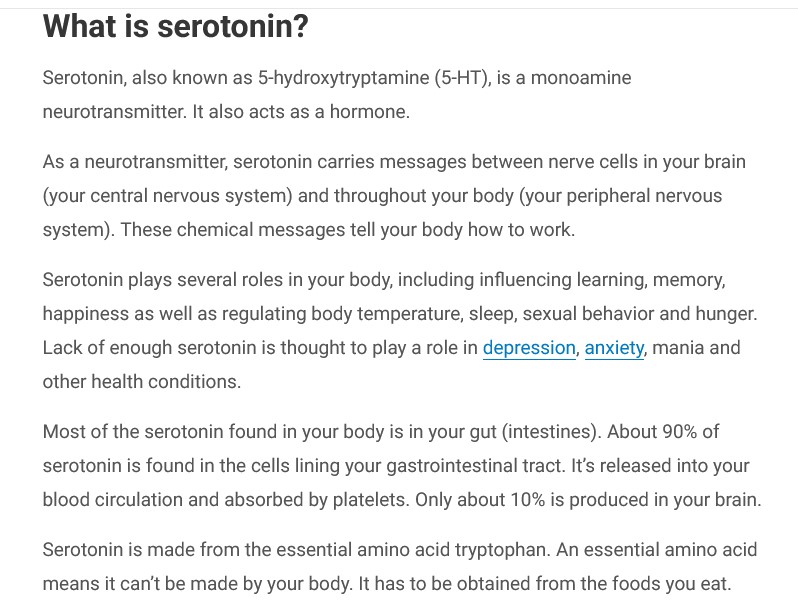
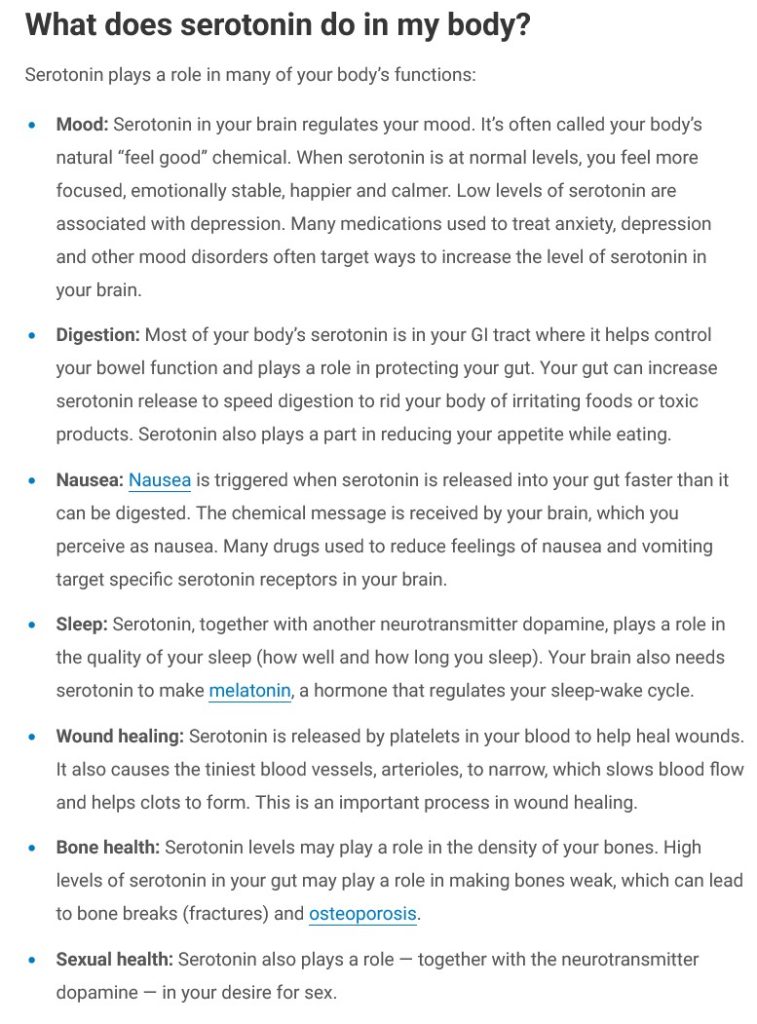
Dr. Ralph Baric has continued to experiment with various types of viruses; among them, coronaviruses and flaviviruses (an example of a flavivirus is the Yellow Fever virus.) Below are screenshots from NIH RePorter regarding a grant to Dr. Baric given in the year 2022 [2]: the Title of the grant; the Abstract; the Funding Information source (NIAID); and, the Other Information about the grant:



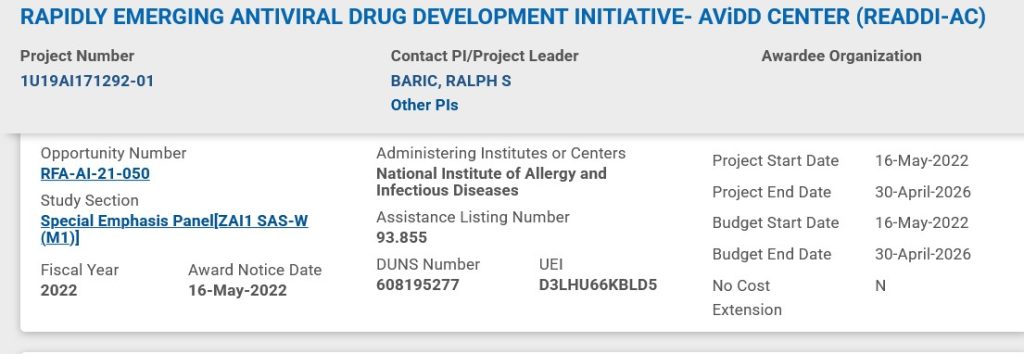
Note the Budget Start Date of 16 May 2022 for the grant. Dr. Anthony Fauci retired from the NIAID (and NIH) at the end of December 2022.
Yours Truly will discuss the READDI-AC initiative, spearheaded by Dr. Baric, further down in today’s offering.
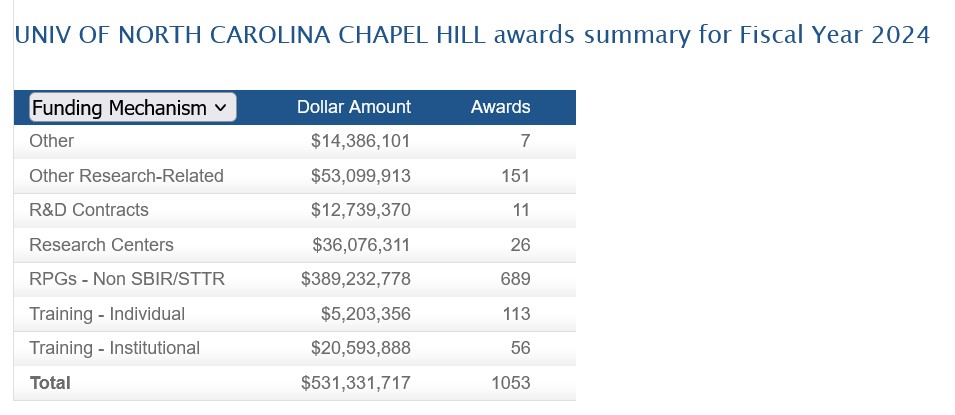
Another example, from the fiscal year 2024, NIH grants to the University of North Carolina, Chapel Hill, per Report.nih.gov/ [3]. Please see the screenshot below:
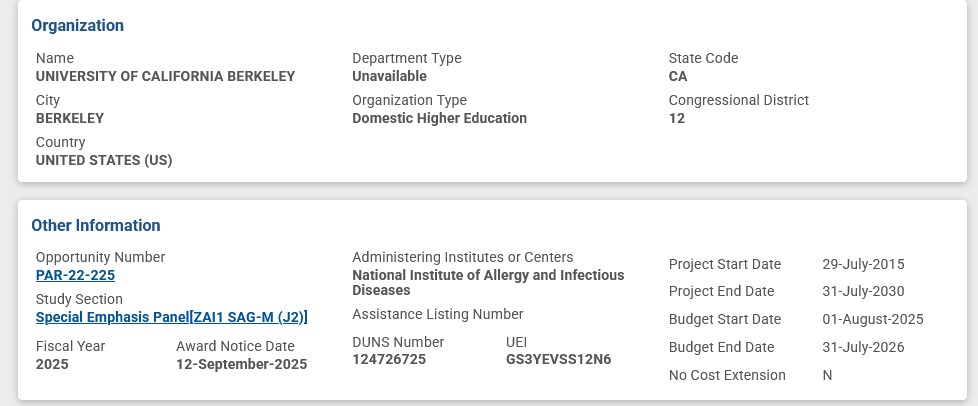
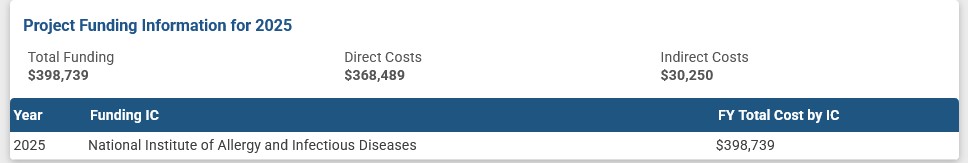
An example of NIH grants to Dr. Baric, this one related to experiments with Dengue Fever, with Dr. Baric being associated with the University of California Berkeley [4]. Please see the screenshots below regarding this grant: the Title; the Abstract; the Funding Institution (NIAID); the Funding grantee institution; and, the funding amounts list:


CIA involvement with Dr. Baric:
The following is from the article by Dr. Peter A. McCullough, MD, MPH, in The Focal Points [5]:

There is a video interview with Dr. McCullough on this topic embedded in the article.
The following screenshots are from the Zero Hedge article by Tyler Durden [6] on this subject (linked in The Focal Points article [5]):



Dr. Baric and his communications: possible “protecting” of him and his communications:
Regarding the possible “protection” of Dr. Baric:
An article by Matt Hartman in The Assembly NC from March 2025 [7] has the following regarding an FOIA request from U.S. Right to Know for emails and other communications by Dr. Baric and the Wuhan Institute of Virology. Please see the screenshot from this article, below:

Another article on the requests for emails and other communications involving Dr. Baric and the Wuhan Institute of Virology, this on by Theresa Opeka in The Carolina Journal, June 2025 [8]. North Carolina Speaker of the state House, Destin Hall, wanted records from 2 July 2020 to 12 August 2024. Please see the screenshot from this article, below:

However, transparency is not, apparently, the priority with the judges of North Carolina. In January 2026, the North Carolina Appeals Court rejected the efforts to allow the public to access the records that the University of North Carolina has regarding Dr. Baric’s interactions with the Wuhan Institute of Virology. This is covered in the article in The Carolina Journal on 7 January 2026. Please see the screenshot from this article, below:

At issue here is the fact that UNC withheld over 5,000 documents related to Dr. Baric and the Wuhan Institute of Virology in response to the original request of US Right to Know. The university did release thousands of copies of other documents in response to the request. USRTK then sued the university to get the withheld documents released. The Appeals Court decision to reject the lawsuit hinges on an interpretation of what is considered to be “proprietary information“, stating that the documents still held by UNC are under “an exemption” to the law in North Carolina. Please see the screenshots, below, from the article on this situation in The Vaccine Reaction, by Carolyn Hendler, JD, 20 January 2026 [10]:

In other words, it appears that the North Carolina Appeals Court did another version of the infamous “what the definition of is, is” of another certain court case of some years ago.
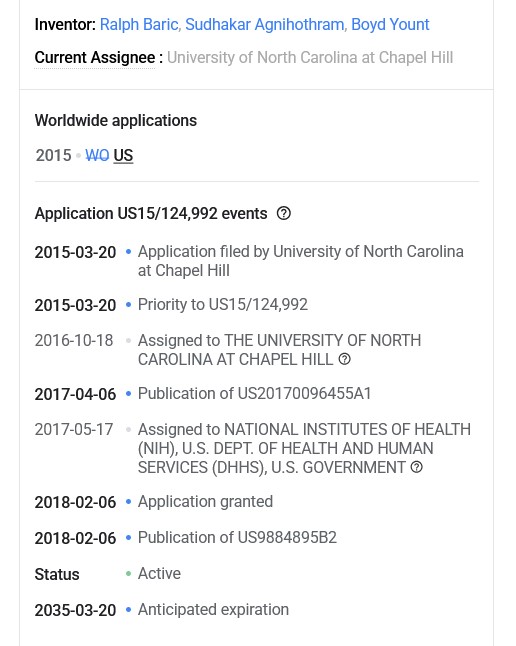
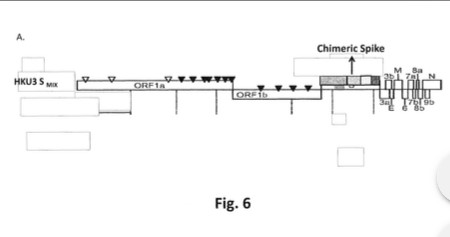
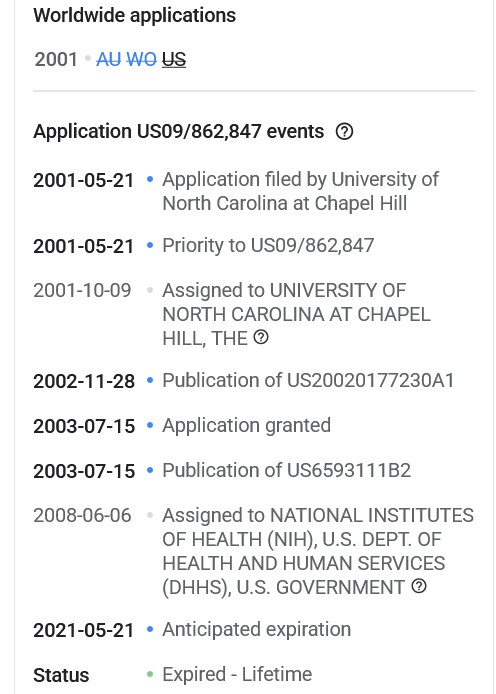
How does this decision (and the court’s interpretation of “proprietary nature” language), square with the fact that Dr. Ralph Baric had already Patented, in March 2015, the SARS-CoV-2 virus “template” that he created in his lab at UNC? This is the Patent Number US9884895B2 that was presented by Yours Truly in The Baric Files, Part Three (see above in today’s offering for the URL link to the post and to the Patent.) And, that the SARS-CoV-2 virus “template” that Dr. Baric lab-created at UNC, and Patented, was based, at least in part, on samples of the SHC014 coronavirus that Dr. Zheng-li Shi (of the Wuhan Institute of Virology) had given to Dr. Baric (at his request) back in 2013? (Again, please refer to the URL link for The Baric Files, Part Three, above in today’s offering.)
One other aspect to all of the above: Dr. Robert Redfield, MD, former Director of the CDC, has stated that the SARS-CoV-2 disaster came from the University of North Carolina, Chapel Hill. Please see the screenshot of the statement by Dr. Redfield from the article by Theresa Opeka, from November 2024, regarding this issue [11]:

READDI-AC:
In 2022, Dr. Ralph Baric established READDI-AC, also known as the AViDD CENTER (READDI-AC), Rapidly Emerging Antiviral Drug Development Initiative – AViDD Center, at the University of North Carolina, Chapel Hill (https://readdi.org/.) This antiviral drug development center was established with a $65 Million dollar grant from NIAID in May 2022. The Funding Information source (NIAID) screenshot from above in today’s offering is repeated, below:

From one of the READDI-AC websites (https://readdi.org/about-readdi/who-we-are):

A screenshot of part of the article from READDI-AC, “Why READDI-AC?” (https://sph.unc.edu/sph/news/why-readdi-inc/), 13 November 2023, is below. Notice that READDI-AC has already “evolved” into a “public-private corporation” of sorts.

A screenshot of the Funders / Collaborators list of READDI-AC (https://readdi.org/about-readdi/partners/) is below:

Dr. Ralph Baric’s wife, Antoinette “Toni” Baric, is the READDI-AC Development Manager. She is, apparently, associated with the UNC Eshelman School of Pharmacy (https://eshelmaninnovation.org/people/; https:/www.linkedin.com/toni-baric-83551628/.)
**** Regarding the collaboration between READDI-AC and the Structural Genomics Consortium (SGC —see above in the READDI-AC Funders / Collaborators screenshot): SGC was the organization that commissioned the 2006 paper by Dr. Baric on Synthetic Genomics — the paper which contained the “outline” and “processes ideas” for lab-creating synthetic genomes for viruses: https://www.jcvi.org/sites/default/files/assets/projects/synthetic-genomics-options-for-governance/Baric-Synthetic-Viral-Genomics.pdf.
The SGC 2007 conference, which featured the ideas in the 2006 commissioned paper by Dr. Baric, is here: https://dspace.mit.edu/bitstream/handle/1721.1/3914/Synthetic%20Genomics%20Options%20for%20Governance.pdf?sequence=1&isAllowed=y. A screenshot of the page in this published conference paper, indicating that Dr. Baric’s 2006 Synthetic Genomics paper was commissioned by the organization, is below (Page 53 of the conference paper):

In Yours Truly’s opinion, it appears that the efforts to “protect” Dr. Baric’s entire communications file from access to the public may be the result of what can be called “take care of me and I’ll take care you”-ism: the University of North Carolina wants its reputation (and its faculty) protected; SGC wants its interests in being the “founding entity” of the lab-created synthetic viral genomics concept protected; NIH / NIAID wants their agencies protected (along with their employees); and, the North Carolina legislature wants its financial stake in READDI-AC protected.
Finally, there is the list of 293 questions for Dr. Ralph Baric paper that was published on ResearchGate: https://www.researchgate.net/publication/397608384_293_QUESTIONS_FOR_DRRALPH_BARIC_2025, also available here: https://doi.org/10.13140/RG.2.2.22713.63840, Billy Bostickson, Steven D. Massey, et al., 14 November 2025. Please see the screenshots of the Brief Background section of this paper, below:



>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>
Yours Truly leaves the reader with the following “core items” related to Dr. Ralph Baric and his research journey, as presented in The Baric Files:
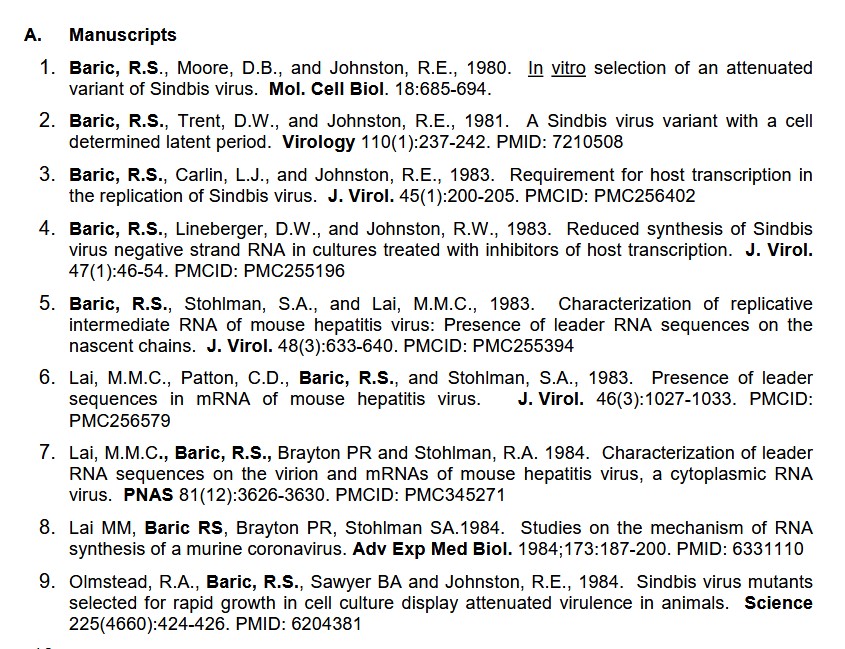
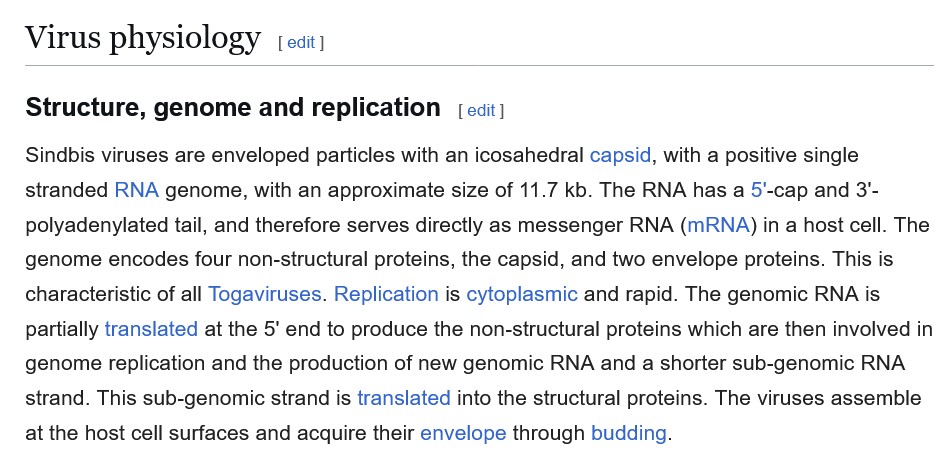
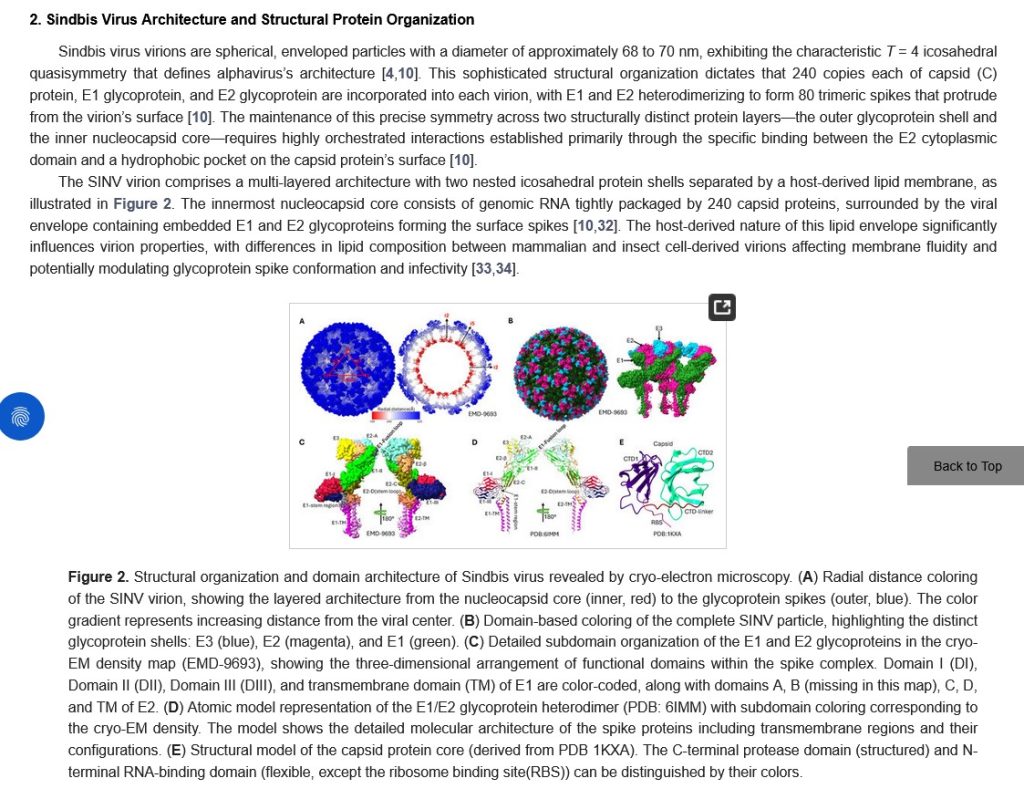
The beginnings of his research journey, the study of the mosquito-borne Sindbis virus;
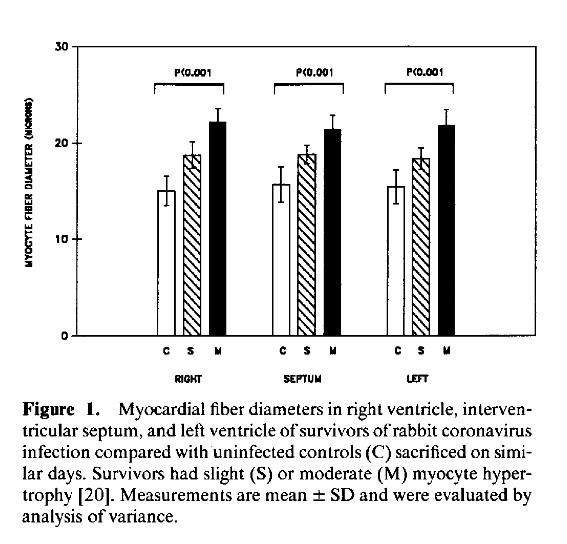
The continuation of his research journey, the study of coronaviruses in mice, rabbits, and pigs;
The continuation of his research journey, the inventions and methods to lab-create animal coronaviruses to study their effects;
The continuation of his research journey, the Synthetic Genomics paper of 2006;
The continuation of his research journey, his collaboration with the Wuhan Institute of Virology;
The continuation of his research journey, his March 2015 Patent of the SARS-CoV-2 virus “template”: Patent Number US9884985B2.
The continuation of his research journey, his November 2015 paper on circulation bat coronaviruses with Dr. Zheng-li Shi, which used the SHC014 bat coronavirus samples that she provided for him;
The continuation of his research journey, the collaboration in the development of the antiviral remdesivir with Gilead Sciences;
The continuation of his research journey, his ongoing efforts since the SARS-CoV-2 virus disaster in experimenting with coronaviruses;
The continuation of his research journey, his co-creation of the EIDD-2801 antiviral treatment with Emory University;
The continuation of his research journey, his establishment of READDI-AC (AViDD) at UNC to lab-create antiviral treatments, in collaboration with (among others) SGC and with the World Health Organization (WHO.)
>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>
THE COVID-19 BIOWEAPON “VACCINES” — ALL OF THEM — MUST BE REMOVED FROM THE MARKET AND FROM ALL USE IN THE UNITED STATES. NOW. PERIOD.
ALL GAIN-OF-FUNCTION RESEARCH MUST BE STOPPED IN THE UNITED STATES. NOW. PERIOD.
IVERMECTIN, HYDROXYCHLOROQUINE, ZINC, VITAMIN C, AND VITAMIN D MUST BE APPROVED FOR BY THE FDA PREVENTION OF COVID-19 VIRUS INFECTION, AND FOR TREATMENT OF COVID-19 VIRUS INFECTION; AND MADE AVAILABLE FOR USE BY THE GENERAL PUBLIC, BY HOSPITALS AND OTHER HEALTHCARE FACILITIES, AND BY HEALTHCARE PROVIDERS. NOW. PERIOD.
THERE. MUST. BE. ACCOUNTABILITY.
THERE. MUST. BE. JUSTICE.
THERE. MUST. BE. TRUTH.
Peace, Good Energy, Respect: PAVACA
Intellectual Disclaimer and Notice: With the exception of published scientific papers, and/or other printed materials, and/or materials available on the internet, the ideas and conclusions of today’s offering are by PAVACA. Proper credit must be given to PAVACA if the ideas and/or conclusions of today’s offering are used by other blog writers, or by podcasters, on social media, or in print media.
Numbered References:
[1]: “Curriculum Vitae Ralph Baric.” https://sph.und.edu/wp-content/uploads/sites/112/2016/09/CV_Ralph_Baric.pdf. September 2019.
[2]: “PI Project Leader – BARIC, RALPH S.” https://reporter.nih.gov/search/sH-_pluTTU25anWQ9nRZcQ/projects.
[3]: “NIH grants to Ralph Baric, 2024.” https://report.nih.gov/award/index.cfm?fy=2024&orgid=578206&om=n.
[4]: “CORE B:Viral Assays Core.” https://reporter.nih.gov/search/9rbNLfGBjU–3je0VeNZtQ/project-details/11111753.ProjectNumber2P01AI106695-11.
[5[: “SARS-CoV-2 Basic Architect Ralph Baric collaborated with CIA.” Peter A. McCullough, MD, MPH. https://www.thefocalpoints.com/p/sars-cov-2-architect-ralph-baric. 28 November 2025.
[6]: “CIA Met With Ralph Baric In 2015 To Discuss “Coronavirus Evolution And Possible Human Adaptation”: Emails.” Tyler Durden. https://www.zerohedge.com/political/cia-met-ralph-baric-2015-discuss-coronavirus-evolution-and-possible-human-adaptation. 12 November 2025.
[7]: “A Chapel Hill Lab Faces New Threats Five Years After COVID-19.” Matt Hartman. https://theassemblync.com/news/education/higher-education/unc-chapel-hill-.ab-baric-five-years-covid-19/. 27 March 2025.
[8]: “Gov Ops requests records about UNC-Chapel Hill coronavirus researcher Baric.” Theresa Opeka. https://www.carolinajournal.com/gov-ops-requests-records-about-unc-chapel-hill-coronavirus-researcher-baric/. 13 June 2025.
[9]: “Judges reject public records case linked to UNC and COVID-19’s origins.” CJ Staff. https://www.carolinajournal.com/judges-reject-public-records-case-linked-to-unc-and-covid-19s-origins/. 7 January 2026.
[10]: “NC Court Upholds Decision to Deny Access to COVID-19 Origins.” Carolyn Hendler, JD. https://thevaccinereaction.org/2026/01/nc-court-upholds-decision-to-deny-access-to-covid-19-origins/. 20 January 2026.
[11]: “Former CDC director claims that COVID-19 emanated from UNC-Chapel Hill.” Theresa Opeka. https://www.carolinajournal.com/former-cdc-director-claims-that-covid-19-emanated-from-unc-chapel-hill/. 18 November 2024.