The above free vintage image of a vaccine vial and syringe is courtesy of iStock and Google Images.
Health Friday is a series devoted to information about Big Pharma, vaccines, general health, and associated topics. As today’s offering is related to the COVID-19 biological toxin injections, aka the COVID-19 “vaccines”, Yours Truly dedicates it to all persons, of whatever age or location, who have been injured, made ill, become disabled, or have passed away, from the negative effects of these “vaccines” that they had in their body.
There are Important Notifications from our host, Wolf Moon; the Rules of our late, good Wheatie; and, certain caveats from Yours Truly, of which readers should be aware. They can be found here. NOTE: Yours Truly has checked today’s post for any AI-generated content. To the best of her knowledge and belief, there is none. If readers wish to post any AI-generated content in today’s discussion thread, they must cite their source. Thank you.
Yours Truly began writing about the results of the huge C4591001 clinical trial of the Pfizer-BioNTech modRNA COVID-19 “vaccine”, BNT162b2, on the board here back in 2023. I was reading through document after document that the company generated related to this clinical trial, documents that were released to the general public only after Pfizer-BioNTech, in partnership with the FDA, lost their case in federal court to keep all of the data about C4591001 sealed for 75 years, and they were then sued by Attorney Aaron Siri’s group, Public Health and Medical Professionals for Transparency (PHMPT.) Please see: https://www.biospace.com/non-profit-group-wins-transparency-lawsuit-over-fda-records-of-pfizer-vaccine-authorization, 7 January 2022. Note: regarding the Pfizer-BioNTech and the Moderna COVID-19 “vaccines”, “mRNA” and “modRNA” are interchangeable descriptive words for these injectables.
The FDA press release of 11 December 2020, announcing the agency’s granting of the EUA for the Pfizer-BioNTech modRNA COVID-19 “vaccine” BNT162b2 is here: https://www.fda.gov/news-events/press-annoucenments/fda-takes-key-action-fight-against-covid-19-issuing-emergency-use-authorization-first-covid-19, “FDA Takes Key Action in Fight Against COVID-19 By Issuing Emergency Use Authorization for First COVID-19 Vaccine.” A screenshot from this press release is below:

Note the phrase, “Follows Thorough Evaluation…”. It is now known that this manifestly was NOT performed before the EUA was granted.
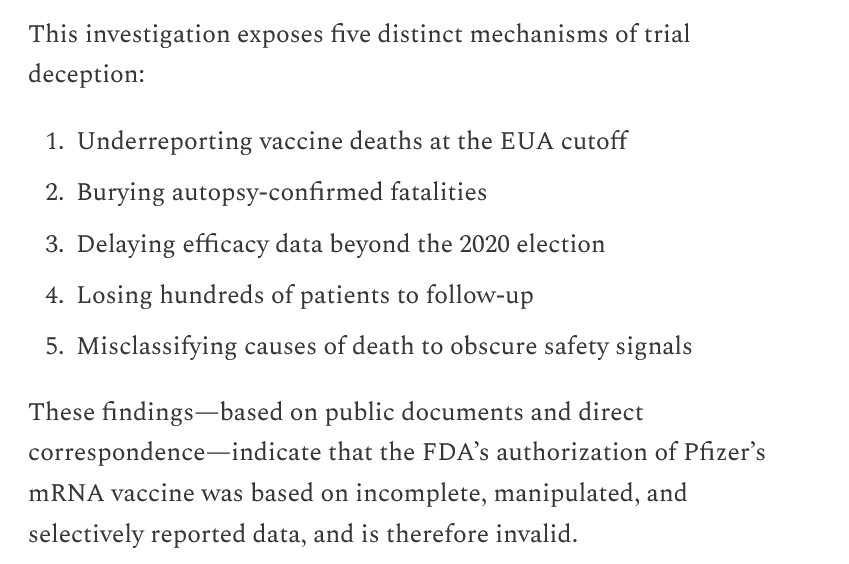
Regarding the invalidity of the 11 December 2020 EUA that was granted to Pfizer-BioNTech for BNT162b2 to be used “to prevent COVID-19 infection” in the United States: Yours Truly begins here: https://www.thefocalpoints.com/p/fda-authorization-of-pfizer-covid. “FDA VRBPAC December 11, 2020 Decision on Pfizer mRNA Found Invalid”, Nicolas Hulscher, MPH, 17 May 2025. There are several screenshots from this article, below:




Regarding the delaying by the FDA and the CDC of important information regarding the incidence of myocarditis following COVID-19 “vaccination”, and these agencies (and, also, Pfizer-BioNTech and Moderna) failing to issue Black Box Warnings about this on the Package Inserts for their modRNA COVID-19 “vaccines” (BNT162b2 [Pfizer-BioNTech] and mRNA-1273 [Moderna]), please see: https://www.thefocalpoints.com/p/us-fda-and-cdc-delayed-health-advisory, “US FDA and CDC Delayed Health Advisory on COVID-19 mRNA Vaccine Myocarditis for Months, Failed to Issue Black Box Warning for Years”, Peter A. McCullough, MD, MPH, 18 May 2025. A screenshot from this article is below:

The above slide image is from the FDA’s VRBPAC meeting of 22 October 2020. This meeting was held seven weeks prior to the 11 December 2020 granting of the EUA for BNT162b2. The FDA therefore KNEW before 11 December 2020 that BNT162b2 could cause myocarditis — but went ahead and issue the EUA anyway.
Yours Truly has written extensively on the manufacturing process for BNT162b2, and on associated topics. Please see: https://www.theqtree.com/2023/11/06/the-infamous-process-2-manufacturing-method-for-the-pfizer-biontech-modrna-covid-19-vaccines/
The FDA’s VRBPAC members will meet on Thursday 22 May 2025 to make “recommendations” regarding the “2025-2026 COVID-19 Vaccine Formulas.” Public comment is accepted until 11:59PM on Friday 23 May. To submit comments electronically, please see: https://www.federalregister.gov/2025/05/08/2025-080803/vaccines-and-related-biological-products-advisory-committee-notice-of-meeting-establishment-of-a; scroll down this page to the section “Electronic Submissions.”
But wait, there’s more! The “new” leadership of the FDA and the CDC, Dr. Vinay Prasad and Dr. Martin Makary wrote an article which was just published in the New England Journal of Medicine: https://doi.org/10.1056/NEJMsb2506929, “An Evidence-Based Approach to COVID-19 Vaccination”, Vinay Prasad, MD, MPH, and Martin A. Makary, MD, MPH, 20 May 2025. This article is NOT an “opinion piece” — Drs. Prasad and Makary make it clear that they are going to implement this “new approach” to COVID-19 “vaccination” through the FDA and the CDC.
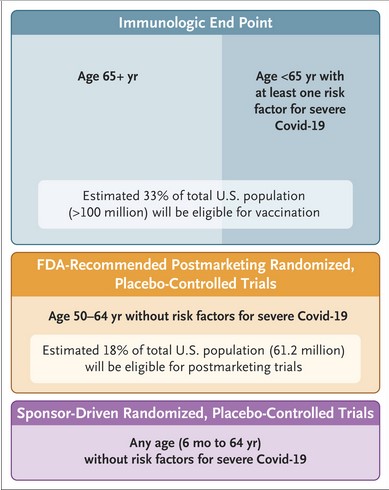
In Yours Truly’s opinion, this “new approach” has many items to question. For example: the granting of FDA authorization for “new formula” COVID-19 “vaccines”, authorization based on lab-performed experiments on the “new formula” ingredients that produce certain numbers of “antibody titers” that might “correspond” to “effectiveness.” There would be no clinical trials at all, performed either on lab rats or on humans. This “lab-experiments with Petri dishes results” authorization method is outlined in “Option 4” of the FDA vaccine authorization / full approval guidelines that the agency adopted in 2022. This “lab-experiments with Petri dishes results” method will now be used for “new formula” COVID-19 “vaccines” for persons age 65 and over; and for persons under age 65 with compromised immune systems or who are part of “vulnerable” or “at risk” populations — such as, for example, pregnant women. Please see, regarding the “Option 4”: https://www.fda.gov/media/159452/download, “VRBPAC Briefing Document”, 28 June 2022. A screenshot of “Option 4” is below:

For another example: COVID-19 “vaccination” will still be “recommended” for pregnant women and for women who have just given birth. This flies in the face of the mounting, and published, evidence that COVID-19 “vaccination” during pregnancy can, and does, result in miscarriages, stillbirths, live births but the infant has medical issues, and so on. In addition, COVID-19 “vaccine” antibodies show up in the breast milk that “vaccinated” new mothers nurse their infants with.
Why do the FDA / CDC continue to ignore the evidence-based facts that Ivermectin, Hydroxychloroquine, Zinc, and Vitamin D both prevent and treat COVID-19 infections?
Three screenshots from the Prasad and Makary article are below:


NOTE THE LAST SENTENCE OF THE ABOVE IMAGE: “Ultimately, these studies alone can provide reassurance that the American repeat-boosters-in-perpetuity strategy is evidence-based.”
Let’s take a look at the combined Figure 2 and Figure 3 image:
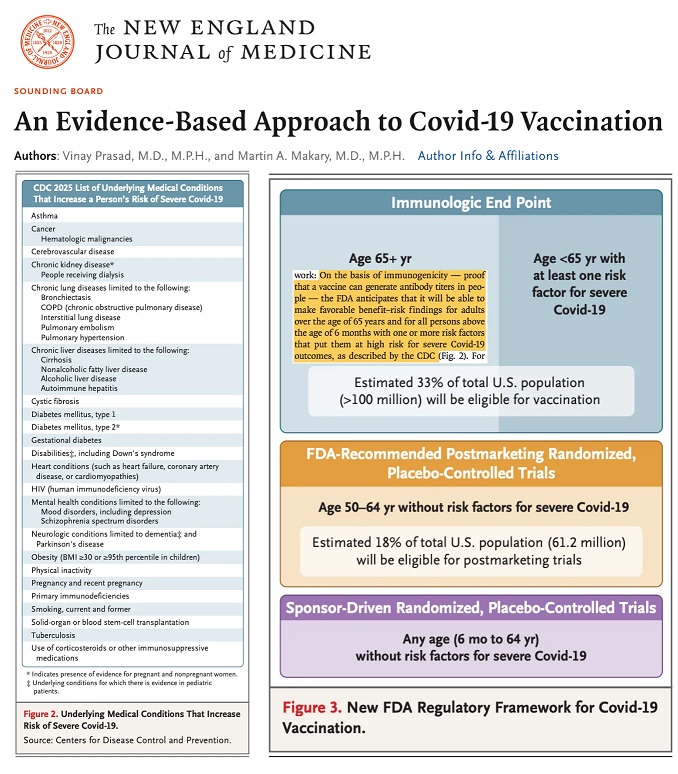
Which makes it plain, in Figure 2, that the COVID-19 “vaccines” will be “recommended” for people who “fit” the diagnosis parameters of multiple types of medical conditions, including pregnant women and women who have just given birth — in other words, these groups of people may well be subjected to multiple types of “convincing” strategies to get them to agree to take these “vaccines.” Who made the decisions on the types of “risk factors” for the “increased at-risk” groups?
And, there’s this tweet, from Dr. Martin Makary, of August 2023:
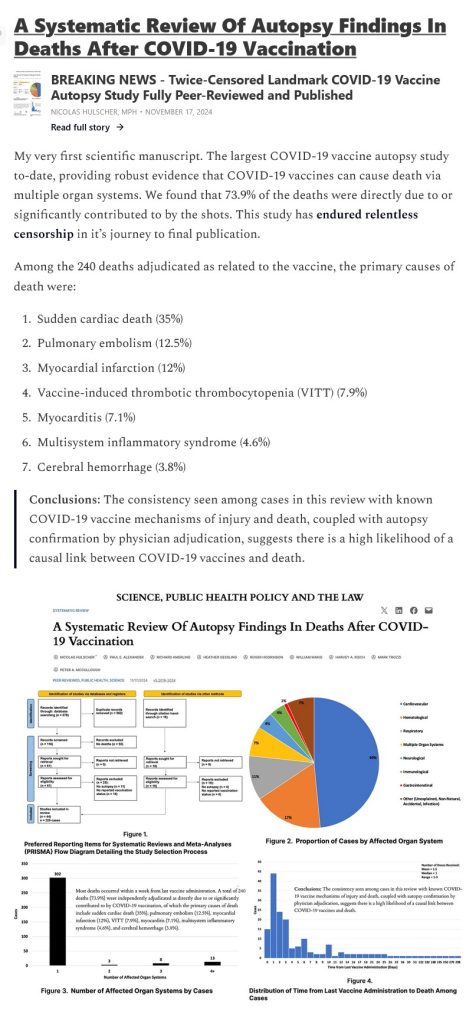
There is published, irrefutable evidence that the COVID-19 “vaccines” can cause death among the “vaccinated.” Please see: https://www.thefocalpoints.com/p/the-causal-link-between-covid-19, “The Causal Link Between COVID-19 Vaccination and Death”, Nicolas Hulscher, MPH, 21 May 2025. There is an embedded interview between Mr. Hulscher and Dr. Idriss J. Aberkane, PhD, on this subject. A screenshot from the Hulscher article is below:
It appears to be unclear, in Yours Truly’s opinion, about where this “new approach to COVID-19 vaccination” fits in as regards the “Generation Gold Standard” that was announced a few weeks ago. Does the federal government control “new” COVID-19 “vaccine” development processes? Where does Big Pharma (Pfizer-BioNTech, Moderna, Novavax) come in? Is that what “Sponsor-Driven” clinical trials means (see the above image)?
However, here’s the real situation: In Yours Truly’s opinion, given that the initial EUA granted by the FDA to the Pfizer-BioNTech BNT162b2 on 11 December 2020 was invalid — that means, by extension, that every other EUA (and “Full Approval”) of the modRNA COVID-19 “vaccines” is also invalid: which would include any “formula” that is “recommended” for the “2025-2026 COVID-19 Vaccine”. Which would also, in Yours Truly’s opinion, invalidate any “Full Approval” of the Novavax COVID-19 “vaccine”, since the foundation of that injectable is the same Wuhan Hu1 SARS-CoV-2 virus that was used as the foundation for BNT162b2.
FLASH! — Meanwhile, the FDA just granted “Full Approval” to the Novavax company’s injectable on 19 May 2025, under the name “NUVAXOVID”: https://ir.novavax.com/press-releases/2025-05-19-U-S-FDA-Approves-BLA-for-Novavax-COVID-19-Vaccine.
FLASH! 2 — The VRBPAC members voted unanimously today to “recommend” that the “2025-2026 COVID-19 Vaccine Formula” injectables contain the JN.1 Omicron variant of the original SARS-CoV-2 virus. This is the same strain that was “recommended” for the “2024-2025 COVID-19 Vaccine Formula” injectables. The decision today by VRBPAC will be implemented according to the Dr. Prasad and Dr. Makary “new approach” method, as outlined above in today’s post. This means that persons age 65 and older, and that persons under age 65 who fall into one of the “increased risk” categories (Figure 2, above in the post) will be “encouraged” to get “vaccinated.” The exact formulation of the “2025-2026 COVID-19 Vaccine Formula” for the Pfizer-BioNTech and the Moderna injectables will be based, as was their other COVID-19 “vaccines” on the modRNA (aka mRNA)-based platform. The Novavax (now called NUVAXOVID) “2025-2026 vaccine” product will be based on the company’s previous “inactivated protein”-based platform. It is unclear whether the NUVAXOVID “2025-2026 vaccine” product will be authorized for persons under age 65 and/or who have underlying “increased risk” conditions. Please see: https://www.cidrap.umn.edu/covid-19/fda-vaccine-advisers-recommend-sticking-jn1-strain-next-covid-vaccines, 22 May 2025; and, https://cen.acs.org/pharmaceuticals/vaccines/FDA39s-new-COVID-19-vaccine/103/web/2025/05?sc=230901_cenrssfeed_eng_latestnewsrss_cen, 22 May 2025. A screenshot from the C&EN / ACS article is below, highlighting items related to the Dr. Prasad and Dr. Makary “new approach” article:

THERE. MUST. BE. JUSTICE.
Peace, Good Energy, Respect: PAVACA