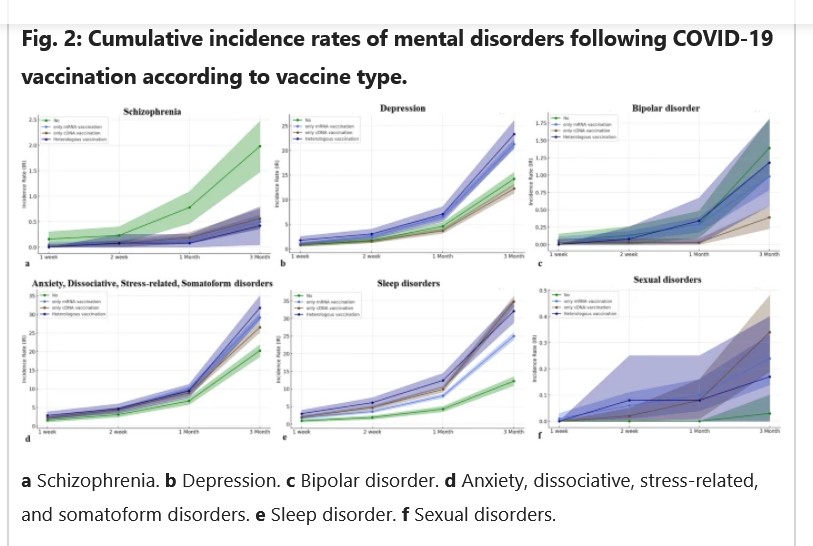
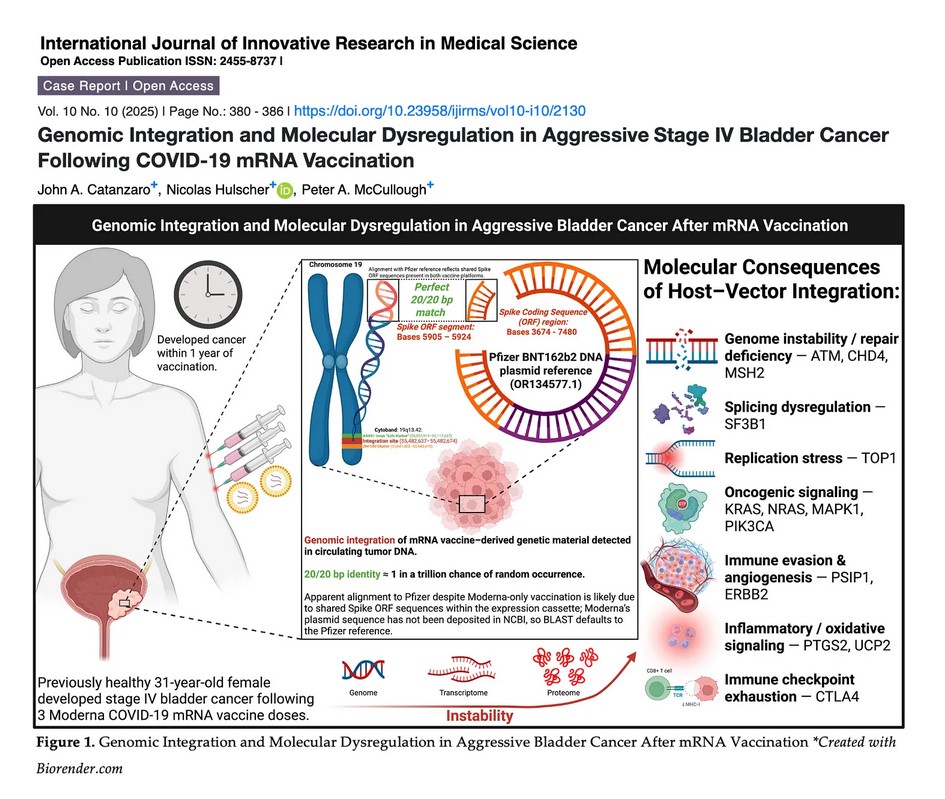
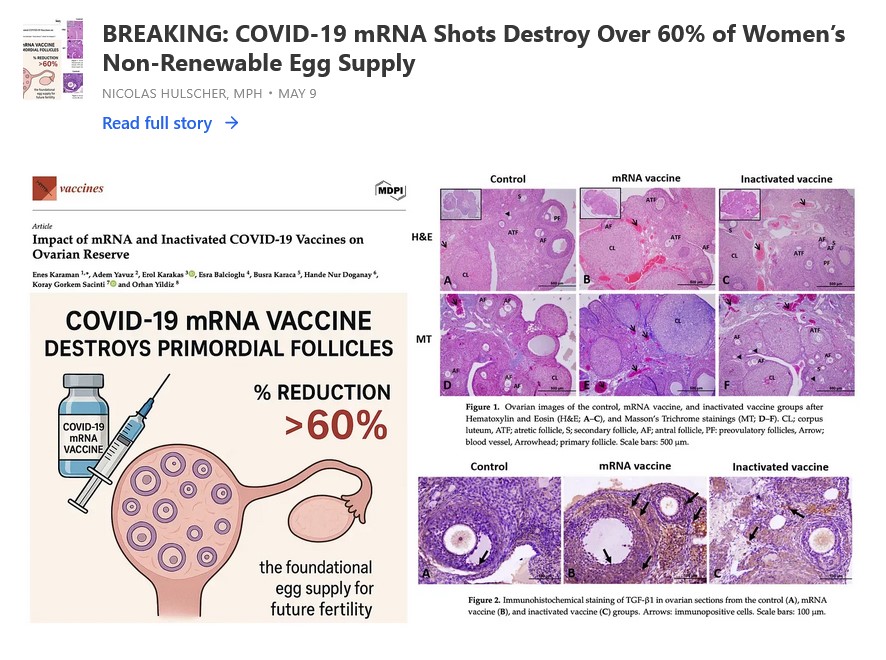
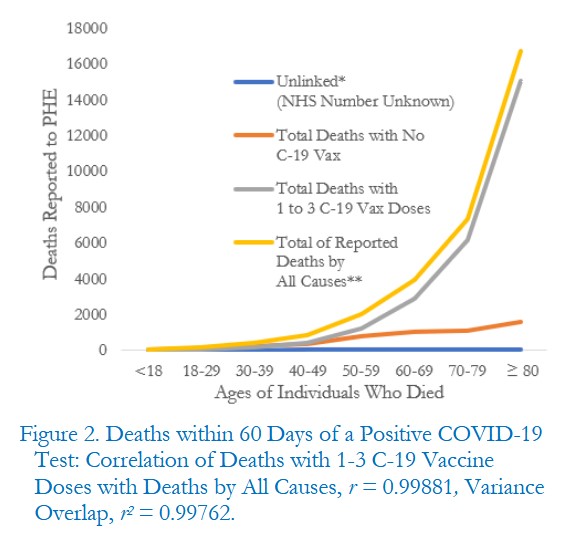
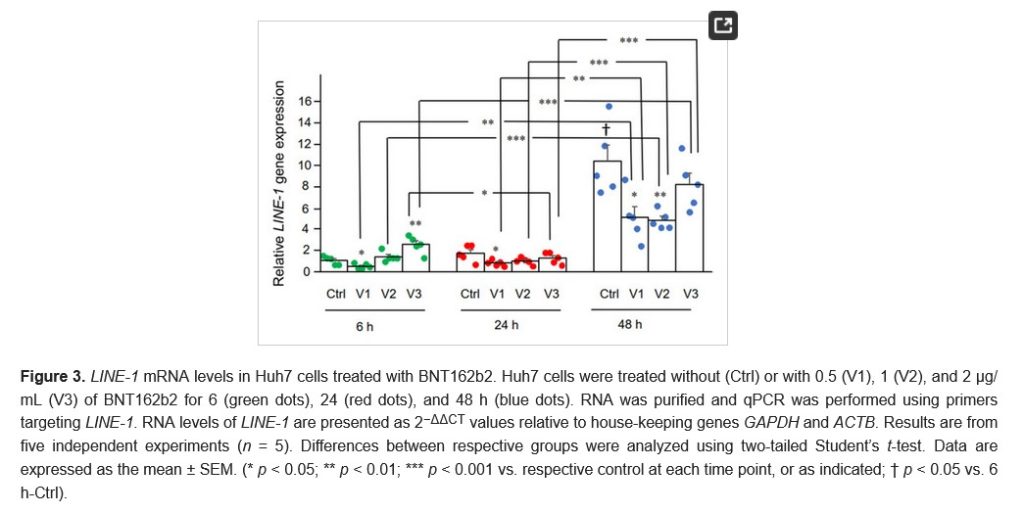
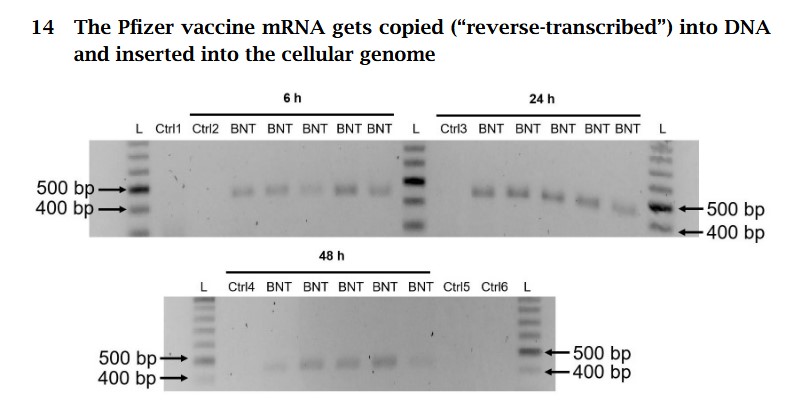
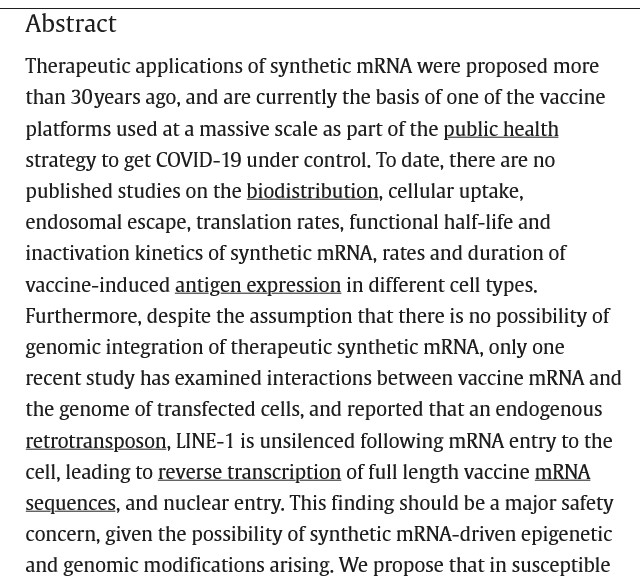
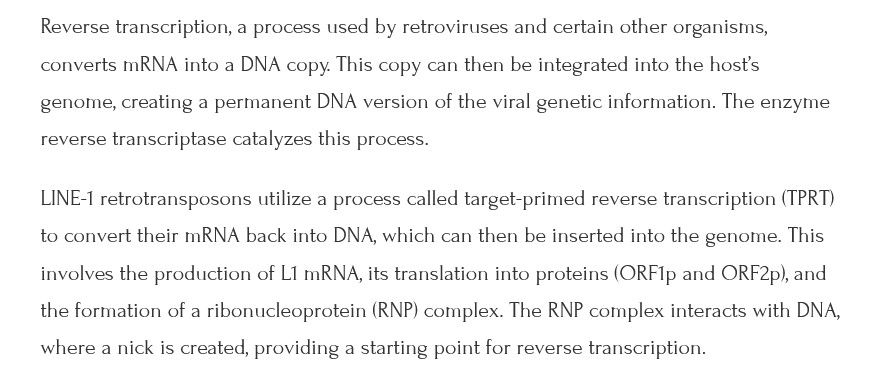
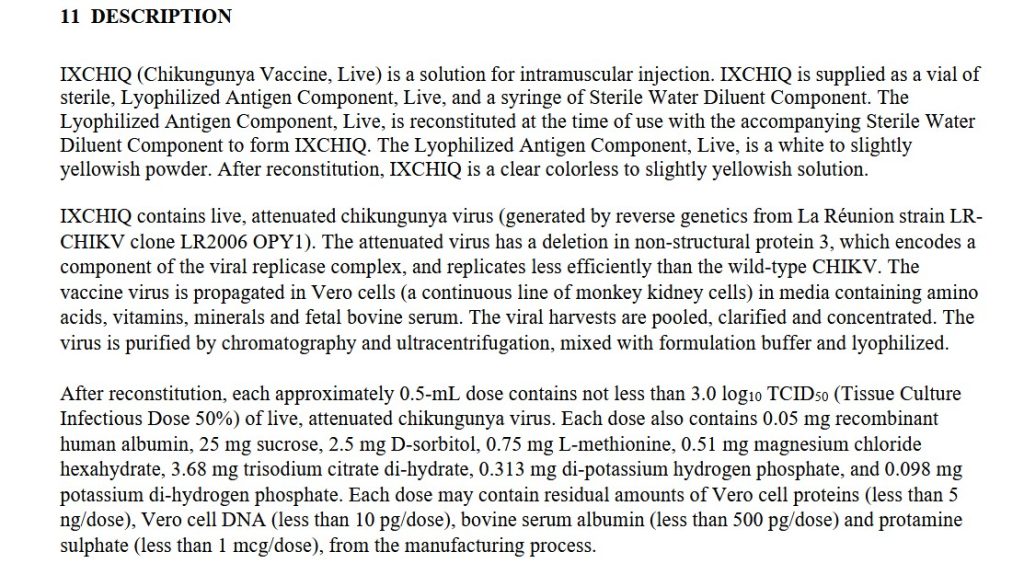
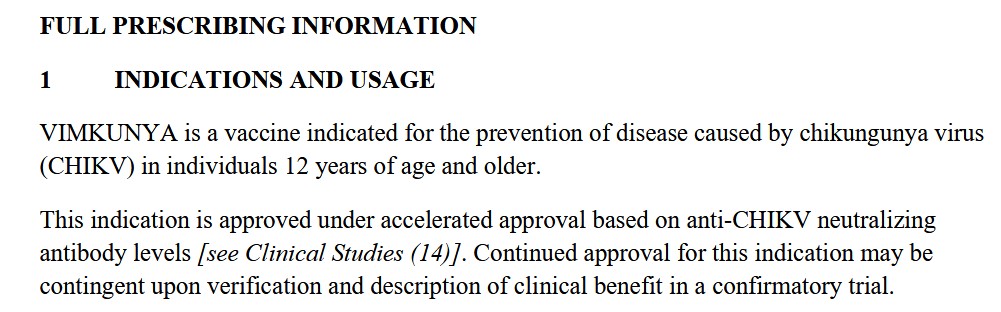
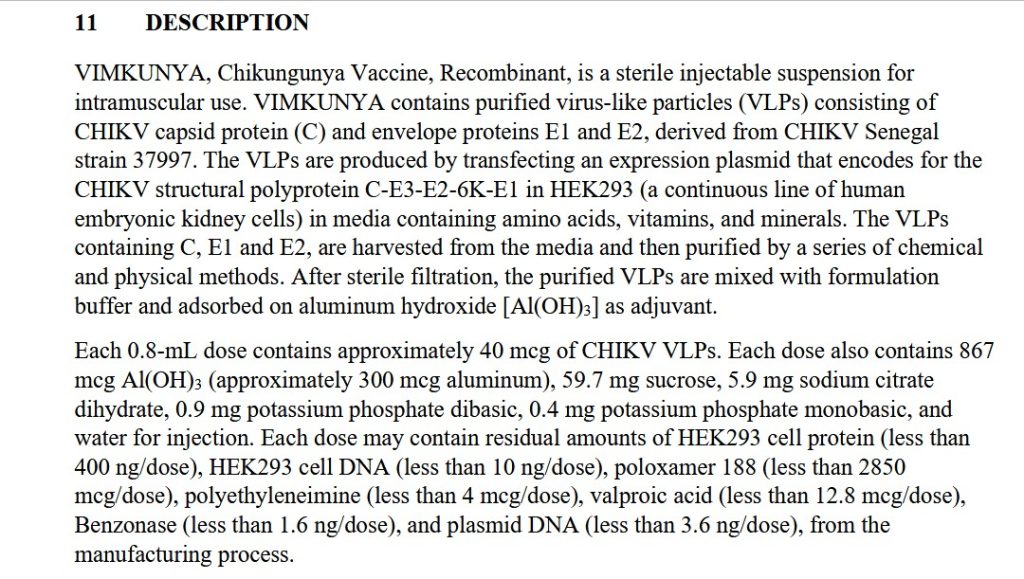
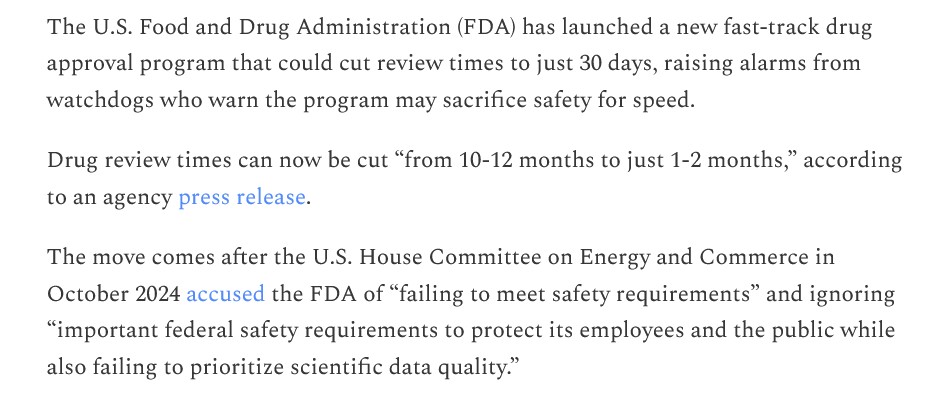
The free image of vintage Pfizer vaccine vials for the header in today’s offering is courtesy of Dreamstime and Google Images.
Health Friday is a series devoted to Big Pharma, vaccines, general health, and associated topics. As today’s offering speaks to the COVID-19 “vaccines”, Yours Truly dedicates it to all persons, of whatever age or location, who have passed away from the negative effects of the COVID-19 “vaccines” that they had in their body. May they rest in eternal Peace.
There are Important Notifications from our host, Wolf Moon; the Rules of our late, good Wheatie; and, certain caveats from Yours Truly, of which readers should be aware. They are linked here. Note: Yours Truly has checked today’s offering for AI-generated content. To the best of her knowledge and belief, there is none, except perhaps for AI-generated images within linked URLS. If readers wish to post AI-generated content to the discussion thread for today’s offering, they must cite their source. Thank you.
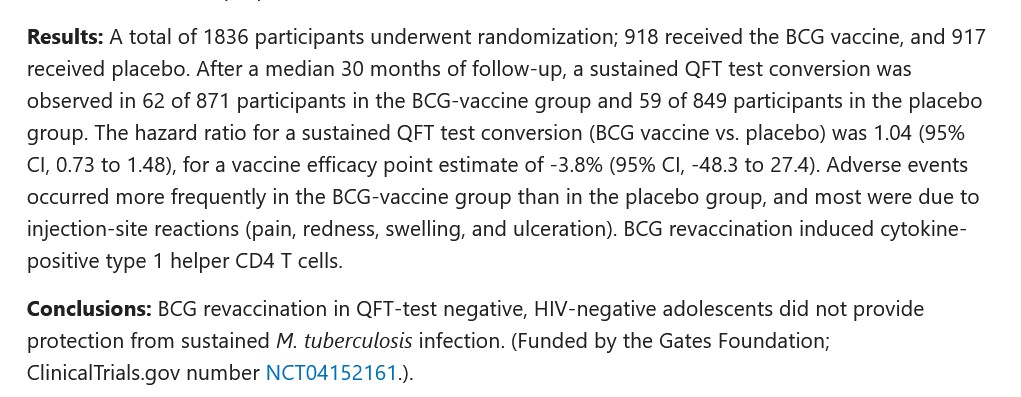
This Part One trail begins here, with two news reports regarding the September 2025 agreement reached between the United States government and Pfizer (PfizerUSA, the United States co-partner of Pfizer-BioNTech; BioNTech, the other co-partner, is headquartered in Mainz, Germany.). First, from Virginia Business (https://virginiabusiness.com/pfizer-agrees-to-lower-drug-costs-70b-us-investment/), “Pfizer agrees to lower drug costs, $70B US investment”, 30 September 2025. A screenshot from this article is below:
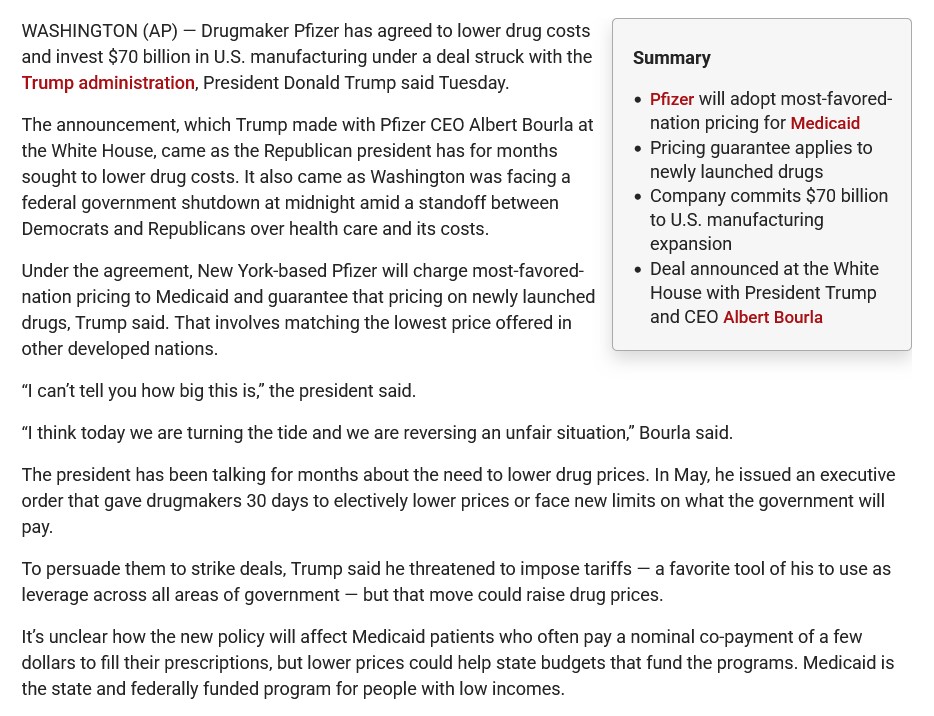
And, second, the announcement from PfizerUSA (https://www.pfizer.com/news/press-release/press-release-detail/pfizer-reaches-landmark-agreement-us-government-lower-drug, “Pfizer Reaches Landmark Agreement with U.S. Government to Lower Drug Costs for American Patients”, 30 September 2025. A screenshot from this article is below:

Note that the specific details of this agreement, which will affect millions of Americans, “remain confidential.”
The company called Pfizer-BioNTech is a formal co-partnership between Pfizer, also known as PfizerUSA (CEO, Dr. Albert Bourla, DVM); and, BIoNTech of Mainz, Germany (CEO, Dr. Ugur Sahin, MD.) Both PfizerUSA and BioNTech were involved / still are involved, in the development and manufacture of the modRNA COVID-19 “vaccine” line, COMIRNATY (this “vaccine” was previously known as BNT162b2, or “Pfizer-BioNTech COVID-19 Vaccine.“) Both PfizerUSA and BioNTech have agreements regarding their sharing royalty payments for COMIRNATY “vaccines” purchased and used throughout the world. PfizerUSA and BioNTech are also involved in the development and manufacture of other drugs and vaccines, either separately or in coordination with each other.
The most recent (as of 5 August 2025) PfizerUSA (and Pfizer-BioNTech) product “pipeline” website is here: https://www.pfizer.com/science/drug-product-pipeline. Click on “Downloadable PDF” to view the entire pipeline. Screenshots of two pipeline areas, Internal Medicine and Vaccines, follow. The first screenshot, Internal Medicine, with discussion by Yours Truly, is below:
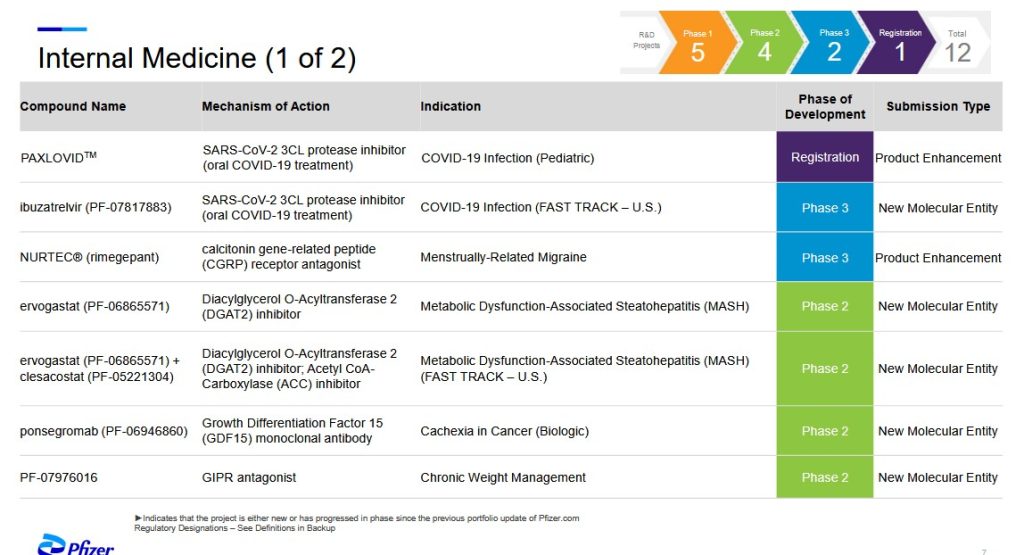
**** WHY is Paxlovid, a combo drug of nirmatrelvir (an antiviral) + ritonavir (an HIV/AIDS treatment drug that targets the immune system) going to be used on CHILDREN who become infected with COVID-19?
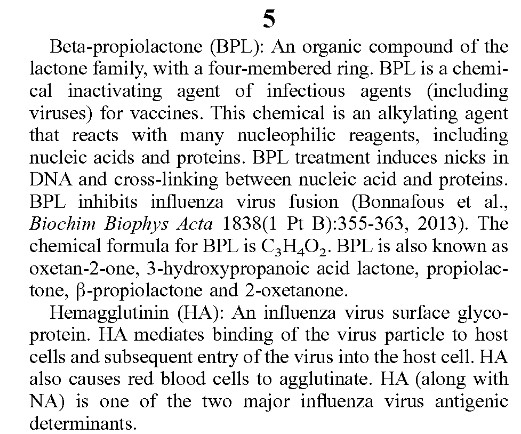
**** Ibuzatrelvir (PF-07817883), now in Phase 3 clinical trials, is an oral / enhanced “nirmatrelvir on steroids” treatment for COVID-19 infection that was granted “Fast Track” approval by the FDA The intended use of this drug appears to be as a “replacement” for Paxlovid.. Please see: https://pubs.acs.org/doi/10.1021/jacsau.4c00508, 30 July 2024. Pfizer has already patented this drug. Let’s take a look at the Overview of this Phase 3 clinical trial, as described here: https://clinicaltrials.gov/study/NCT06679140:
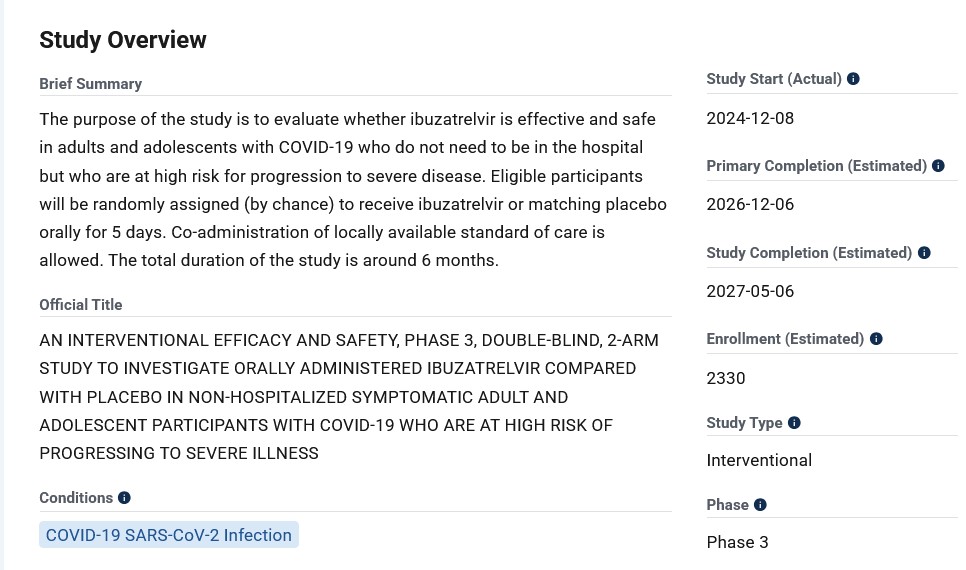
Does “Fast Track” approval by the FDA mean that the “Study Completion (Estimated)” of 6 May 2027 will be “bypassed”, and only the data from the “Primary Completion (Estimated)” of 12 December 2026 will be used to push this drug onto the market faster? In addition, WHY is the study subject pool so small (2330 persons) for a drug that would potentially be used on hundreds of thousands of persons?
And, from the Researcher View of this clinical trial, part of the Secondary Outcomes descriptions:

Note that the “viral load” measurement will be performed via EITHER a nasal sample, OR via a nasopharyngeal sample. If a nasopharyngeal sample swab is used, this is the extremely long swab that reaches all the way to the VERY BACK of the nasal cavity and can touch the COVERING OF THE BRAIN. By the way, the correct administration of the nasopharyngeal swab is also to ROTATE the swab a couple of times after insertion.
In addition, in the information about NCT06679140, the “placebo tablet” that will be used is NOT described at all. Is the “placebo tablet” going to be Paxlovid? Is the “placebo tablet” going to be a completely drug-free “empty” tablet?
And now, the second screenshot from the “pipeline” PDF, Vaccines, is below. Yours Truly will discuss an interesting new “vaccine” from this list, under development by PfizerUSA (in conjunction with BioNTech) — PF-07926307, a combination modRNA-based COVID-19 plus influenza “vaccine“:
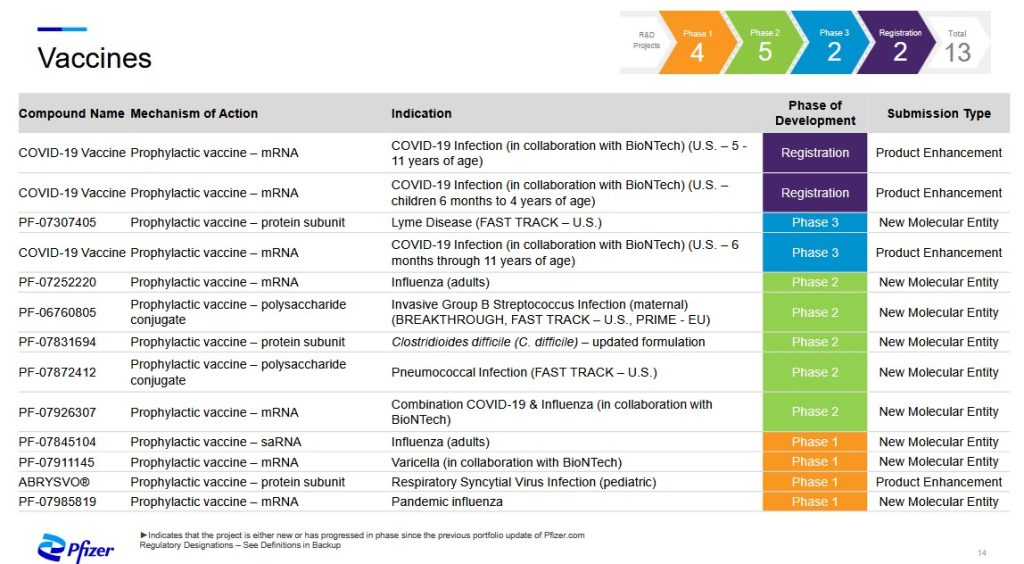
The available-to-the-public information regarding PF-07926307 is both confusing and concerning. Pfizer-BioNTech insists that this “combo vaccine” is only for “prophylactic” use against COVID-19 plus influenza: https://investors.biontech.de/news-releases/news-release-details/pfizer-and-biontech-provide-update-mrna-based-combination, “Pfizer and BioNTech Provide Update on mRNA-based Combination Vaccine Program Against Influenza and COVID-19 in Individuals 18-64 Years of Age”, 16 August 2024. Only ONE of of the two outcomes measurements of the Phase 3 clinical trial for this “vaccine” were met (NCT06178991.) A screenshot from the company’s press release is below:

Note that this press release emanates from Germany, not the United States;, and that the “combination candidate” is not identified as PF-07926307.
**** In addition, it appears that PF-07926307 is actually a combination of TWO separate Pfizer-BioNTech modRNA “vaccines” formulations: BNT162b2 and BNT 161: https://investors.biontech.de/news-releases/news-release-details/biontech-outlines-2024-strategic-priorities-42nd-annual-jp, “BioNTech Outlines 2024 Strategic Priorities at the 42nd Annual J.P. Morgan Healthcare Conference”, 9 January 2024. Please see the screenshot from this article, below:
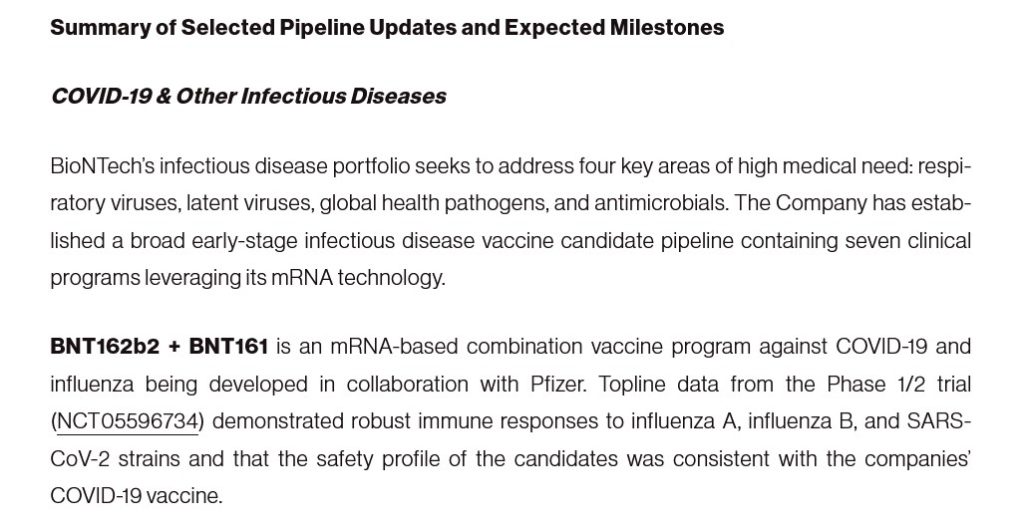
**** Note the clinical trial mentioned in the above screenshot: NCT05596734. The modRNA “combo vaccine” used in this clinical trial is none other than BNT162b2 (tozinameran, now marketed as COMIRNATY, but which was the ORIGINAL Pfizer-BioBNTech modRNA COVID-19 “vaccine” against the ORIGINAL Wuhan Hu1 SARS-CoV-2 [COVID-19] virus), plus BNT161 (famtozinameran, the ORIGINAL modRNA COVID-19 “vaccine” against the OMICRON variant BA.4/BA.5) Please see: https://covid-vaccine.canada.ca/comirnaty-original-omicron-ba4ba5/product-details, which also states, “Cancelled by sponsor May 3rd, 2024.”
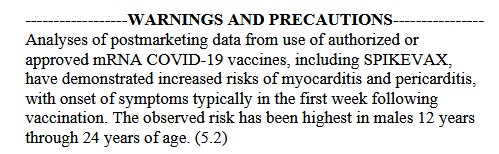
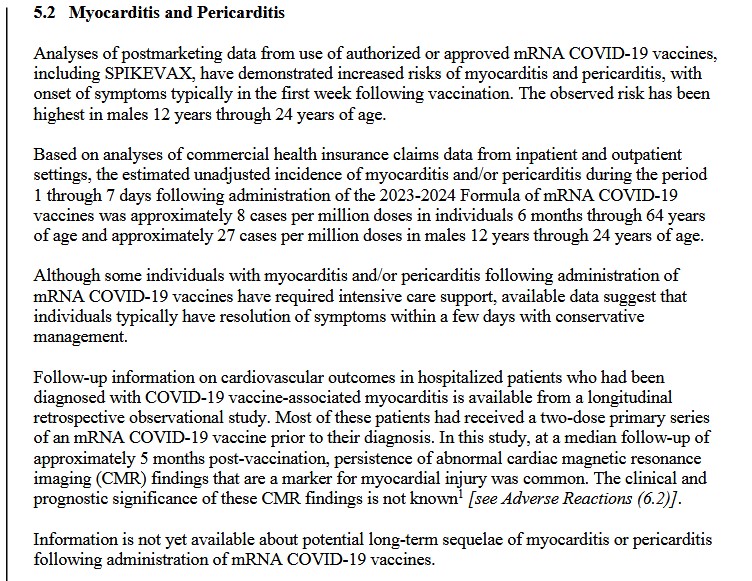
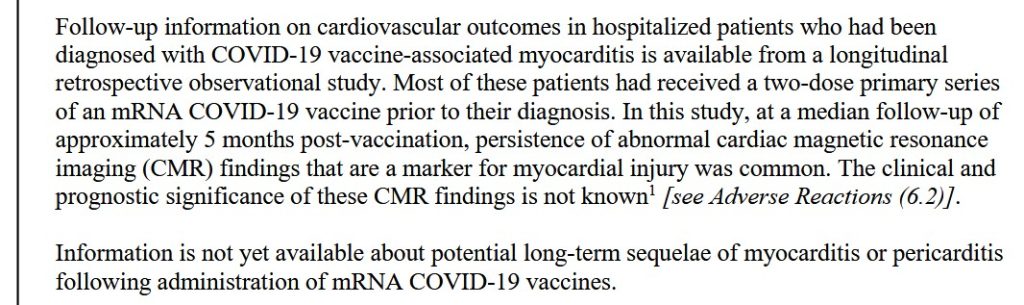
**** However, at the same time, it appears that this Pfizer-BioNTech modRNA COVID-19 “combo vaccine” of BNT162b2 plus BNT161 — also known as PF-07926307 — IS being used — in Singapore: https://labeling.pfizer.com/ShowLabeling.aspx?id=20959, “Date of last revision: July 2024.” Please see the screenshot from the package information for this product administered in Singapore, below:
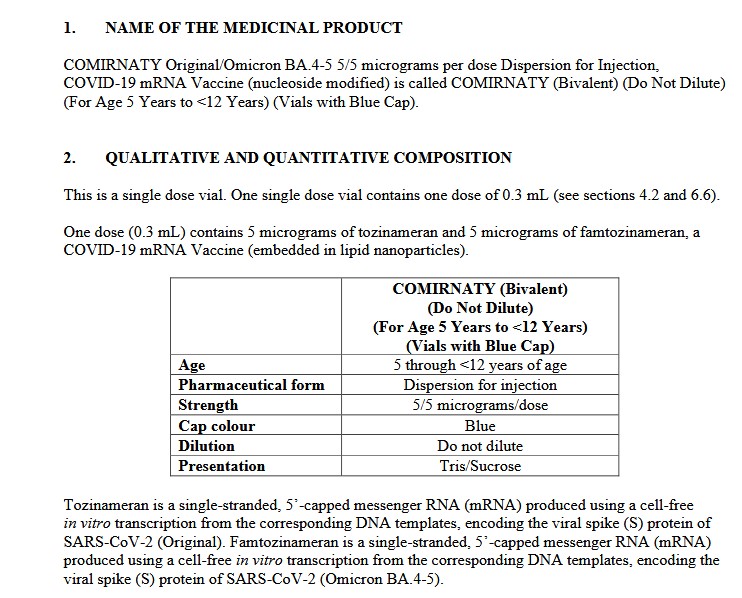
And, screenshots from Page 65 (of 65), from the package information for the above injectable:


BNT161, one of the component modRNA “vaccines” in PF-07926307, is an influenza “vaccine”, meaning that it can be used against EITHER influenza OR COVID-19 (Omicron BA.4/BA.5.) The German partner of PfizerUSA — BioNTech — has been working on this injectable since at least 2022: https://biontechse.gcs-web.com/news-releases/press-release-details/biontech-announces-third-quarter-2022-financial-results-and, “BioNTech Announces Third Quarter 2022 Financial Results and Corporate Update”, 7 November 2022. A screenshot from this article is below:

Note that the press release is from BioNTech Sweden.
A screenshot from the Adisinsight Drug Profile for BNT161 is below. Note that this modRNA “vaccine” is a quadrivalent influenza injectable (https://adisinsight.springer.com/drugs/800052769):

**** On the other hand, here is the article on PF-07926307 (BNT162b2 + BNT161) by the tech / AI / data collection and analysis platform, Patsnap: https://synapse.patsnap.com/article/what-is-pf-07926307-used-for?, 28 June 2024. A screenshot of the entire article is below; Yours Truly includes the entire article because it has a wealth of information and clues as to the possible real “agenda” behind this injectable:

**** Note that, per the article above, the “primary indication” for the use of PF-07926307 is for the treatment of cancers caused by overactive kinases responses, such as are found in lymphomas; with what may be called a “secondary indication” for treatment of chronic inflammatory diseases. Lymphomas or chronic inflammatory diseases induced by, say, modRNA COVID-19 “vaccine” injections, such as BNT162b2 (COMIRNATY?) Is it remotely possible that PF-07926307 (BNT162b2 + BNT161) is being redesigned as a “backdoor oncology and/or chronic inflammatory diseases treatment” injectable? How does this square with what Pfizer-BioNTech claims that this “vaccine” is to be used for — against COVID-19 + influenza infection? What is the truth here?
**** In any case, WHY is Pfizer-BioNTech apparently using BNT162b2, the company’s original modRNA COVID-19 “vaccine”, as a foundational component in the development of a “New Molecular Entity” called PF-07926307, which is to used as a “prophylactic” against COVID-19 plus influenza? What happened regarding all those other modRNA COVID-19 “vaccines” made by this company, to be used against the LATEST MUTATIONS of the SARS-CoV-2 virus, such as the “2025-2026 version” of COMIRNATY? Why is Pfizer-BioNTech going all the way back to the ORIGINAL Wuhan Hu1 SARS-CoV-2 virus contained in BNT162b2 (COMIRNATY) to formulate PF-07926307?
What is going on at Pfizer-BioNTech? Does the United States government know about PF-07926307 (BNT162b2 + BNT161)? Did the “Specific terms of the agreement remain confidential” regarding the deal between the United States government and PfizerUSA reached in September 2025 include provisions that our government “looks the other way” about the activities of PfizerUSA’s co-partner, BioNTech? Does the HHS / FDA / CDC / BARDA have the complete details of the “Specific terms of the agreement remain confidential” provisions? Are the “specific details” being shared with only the “top brass” of the FDA / CDC / BARDA — and that HHS Sec. Kennedy, Jr., is being kept in the dark?
———————————————————————————
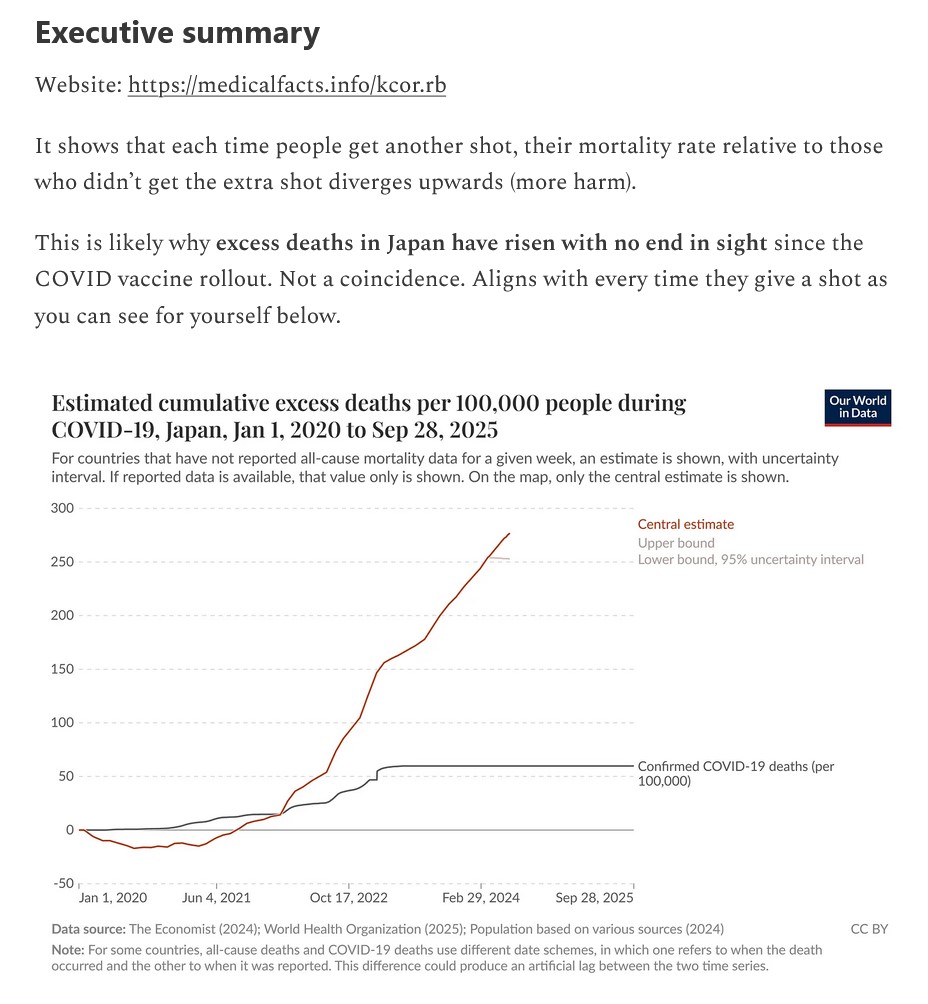
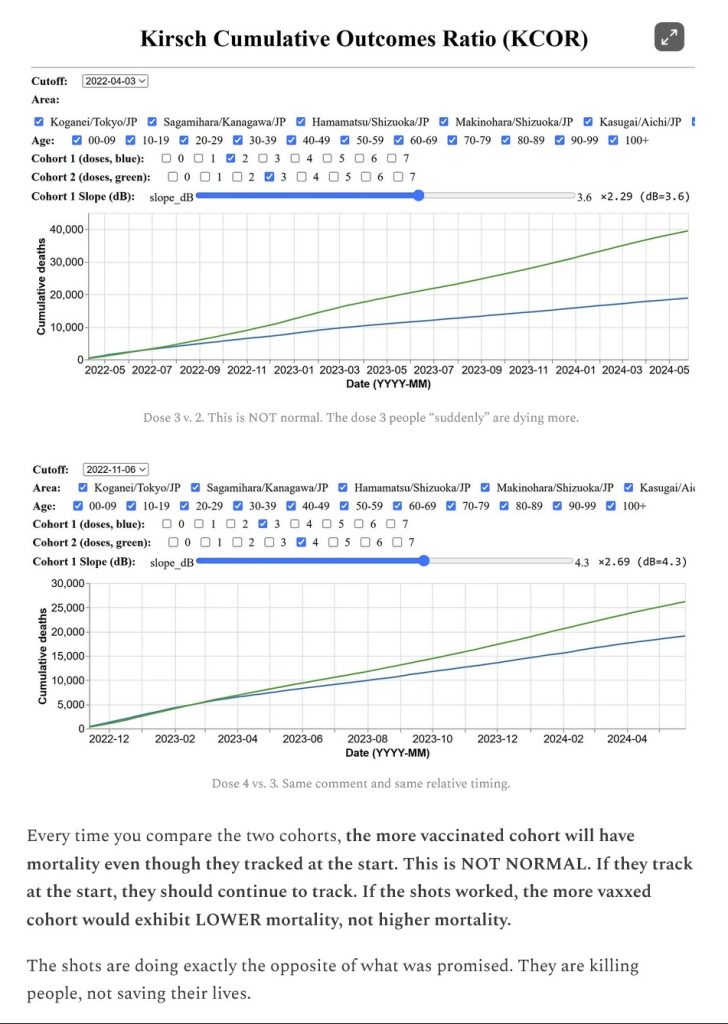
All current COVID-19 “vaccines” — ALL of them — MUST be pulled off the market and from use. Now.
All research and development of “new” COVID-19 “vaccines” that are in ANY form — injectable; oral; nasal; micro-needle — MUST be stopped. Now.
There MUST be complete analysis of the ingredients and mechanisms of the current COVID-19 “vaccines”, performed by impartial testing entities, and with complete results made public. Now.
THERE. MUST. BE. ACCOUNTABILITY.
THERE. MUST. BE. JUSTICE.
THERE. MUST. BE. TRUTH.
Peace, Good Energy, Respect: PAVACA
(Intellectual Property Notice: With the exception of links to published media reports and links to published scientific papers, the ideas and conclusions of today’s post are by PAVACA. Proper credit must be given to PAVACA if other blog writers, or persons on podcasts, social media, or print media, use the ideas and/or conclusions of today’s post. Thank you.)