His Fraudulency
Joe Biteme, properly styled His Fraudulency, continues to infest the White House, we haven’t heard much from the person who should have been declared the victor, and hopium is still being dispensed even as our military appears to have joined the political establishment in knuckling under to the fraud.
One can hope that all is not as it seems.
I’d love to feast on that crow.
Justice Must Be Done.
The prior election must be acknowledged as fraudulent, and steps must be taken to prosecute the fraudsters and restore integrity to the system.
Nothing else matters at this point. Talking about trying again in 2022 or 2024 is hopeless otherwise. Which is not to say one must never talk about this, but rather that one must account for this in ones planning; if fixing the fraud is not part of the plan, you have no plan.
Lawyer Appeasement Section
OK now for the fine print.
This is the WQTH Daily Thread. You know the drill. There’s no Poltical correctness, but civility is a requirement. There are Important Guidelines, here, with an addendum on 20191110.
We have a new board – called The U Tree – where people can take each other to the woodshed without fear of censorship or moderation.
And remember Wheatie’s Rules:
1. No food fights
2. No running with scissors.
3. If you bring snacks, bring enough for everyone.
4. Zeroth rule of gun safety: Don’t let the government get your guns.
5. Rule one of gun safety: The gun is always loaded.
5a. If you actually want the gun to be loaded, like because you’re checking out a bump in the night, then it’s empty.
6. Rule two of gun safety: Never point the gun at anything you’re not willing to destroy.
7. Rule three: Keep your finger off the trigger until ready to fire.
8. Rule the fourth: Be sure of your target and what is behind it.
(Hmm a few extras seem to have crept in.)
Spot Prices.
Kitco Ask. Last week:
Gold $1893
Silver $27.91
Platinum $1172
Palladium $2890
Rhodium $21,000
This week, markets closed as of 3PM MT.
Gold $1877.40
Silver $28.02
Platinum $1153.00
Palladium $2854.00
Rhodium $22,000.00
Gold was actually just below $1900 at open. The others have changed even less on a percentage basis. Since rhodium didn’t just jump right back up to nearly $30K, I’m thinking this price might not be a short term “spike” (but downward).
(Be advised that if you want to go buy some gold, you will have to pay at least $200 over these spot prices. They represent “paper” gold, not “physical” gold, a lump you can hold in your hand. Incidentally, if you do have a lump of some size, doesn’t it give you a nice warm feeling to heft it?)
The Atom
(Part VII of a Long Series)
Introduction
The general outline of this story is to start off by putting you “in touch” with the state of physics at the beginning of 1895. Physicists were feeling pretty confident that they understood most everything. Sure there were a few loose ends, but they were just loose ends.
1895 marks the year when people began tugging at the loose ends and things unraveled a bit. In the next three years, three major discoveries made it plain there was still a lot to learn at the fundamental level.
Once I’m there I will concentrate on a very, very small object…that ties in with stars, arguably the biggest objects there are (galaxies are basically collections of stars). And we would never have seen this but for those discoveries in the 1890s.
It’s such a long story I decided to break it down into pieces, and this is the seventh of those pieces. (Though to be sure this series seems to have taken on a life of its own.)
And here is the caveat: I will be explaining, at first, what the scientific consensus was in 1895. So much of what I have to say is out of date, and I know it…but going past it would be a spoiler. So I’d appreciate not being “corrected” in the comments when I say things like “mass is conserved.” I know that that isn’t considered true any more, but the point is in 1895 we didn’t know that. I will get there in due time. (On the other hand, if I do misrepresent the state of understanding as it was in 1895, I do want to know it.)
Also, to avoid getting bogged down in Spockian numbers specified to nine decimal places, I’m going to round a lot of things off. I used 9.8 kg m/s2 in Part for a number that’s actually closer to 9.80665, for instance, similarly for the number 32. In fact, I’ll be rounding off a lot today.
NOTE: A YUUUGE debt is owed here to “Discovery of the Elements,” 2nd edition, by James L. Marshall.
Why Talk About Atoms?
This post is going to seem like it actually is about chemistry, and in many ways it is.
However, physics and chemistry are right next door to each other. Physics is the most fundamental of the sciences, the others build on it, with chemistry being the one directly “on top” of the physics foundation. Thus it’s the major branch of science most directly connected to physics. And you’ll see some of that here. (Of course, where we divide sciences into major branches is largely arbitrary. For example, if there were a major branch for electricity and magnetism, it’d be tied even closer to physics than chemistry is, but in fact, E&M is considered a branch of physics rather than a major science in its own right.)
Phlogiston
Our story begins with Georg Ernest Stahl, in the 1600s. Before he came on the scene, what we now think of as chemistry was still under the sway of the alchemists, many of whom were trying to turn lead (and other base metals) into gold.
They had a basic theory of chemistry, to wit that the world was made of exactly four basic substances, earth, water, air, and fire (and in some cases they believed the heavens were made out of “aether”, something not encountered “down here”). Everything we see around us, they maintained, was some sort of mixture of these basic elements. So to change lead into gold, all one needed to do was change the mixture, removing some things and adding others.
Of course, that never came to anything, but during all their efforts they amassed a huge amount of knowledge about what would happen if you mixed certain things together and treated them in certain ways.
For example you could mix potash and sulphur, and create liver of sulphur. But you could also create liver of sulphur by heating vitriolated tartar together with charcoal.
(I use the older names here so that you can see how totally arbitrary this must have seemed to the people who used those names.)
So what we had by the 1600s was a vast collection of information like this, with no real way to connect the pieces and understand what was really going on.
And this is where Georg Ernest Stahl comes in.
He was the first to put forward a theory that seemed to tie this disparate trivia together. The theory could also be used to make predictions about what would happen with previoiusly untried processes. This would help tremendously if the theory were right, but would also, if the theory were wrong, allow it to be discredited because it had made a specific prediction that hadn’t come to pass.
(A lot of things people believe are “unfalsifiable.” That means there’s no way, even in principle to disprove it even if it’s wrong. Most real “conspiracy theories” are like this, actually; if any evidence is turned up against the theory, the advocates will dismiss it as falsified as part of the cover up. If your hypothesis can explain away anything this way, and you can disregard evidence against your hypothesis, you can’t be convinced it’s wrong, and the theory itself is worthless since it can be neither proved or disproved, and can make no meaningful predictions, either–any outcome can be made to fit the theory, so any outcome is possible if the theory is true.)
So here it is: Stahl noted some similarities between combustion (burning things), calcining (rust, corrosion), and respiration (both plant and animal “breathing”). He concluded that at a very basic level three of these four were the same thing–he excepted plant respiration, but claimed that it was fundamentally animal respiration in reverse.
His proposed explanation for all of these processes? That wood, when it burns, and metal when it rusts, and animals when they breathe, all give off a substance called phlogiston. Thus, the calx of some metal, say iron rust was a purer substance than the metal, because the metal had given up phlogiston to turn into the rust. Similarly, when you burned a log you could even hear it hiss as the plogiston was released.
The ancients had believed that fire, once released, went up into the heavens; Stalh believed that plogiston combined with the atmosphere, to form phlogisticated air.
Plants would simply recapture the phlogiston from the air, turning the air into dephlogisticated air, and incorporate it into their tissues, forming a sort of closed cycle, ready to be burned again, or eaten by an animal that would breathe and release the phlogiston into the atmosphere once again.
OK, there were a couple of simple objections to this. Wood, when burned would lose weight, but metals, when rusting, gained weight. But it was readily noted that burning wood released a lot of smoke, which surely weighed something, and it was presumed there was a weight gain there, too (which in fact is the case). It was proposed, therefore, that phlogiston had negative weight. This was a concrete prediction of the theory, that phlogiston, if isolated, would have what amounted to antigravity, or as they called it back then, levity.
Chemists made a bunch of progress in the 1770s towards proving this theory and bringing some order to chemistry, much like Newton had done with mechanics a century earlier.
Unfortunately things fell apart under the weight of too much evidence. Too much special pleading had to occur to explain away anomalies.
Phlogisticated air was produced by Daniel Rutherford in 1772. He would burn a candle in a closed container, let a mouse asphyxiate (which took about 15 minutes) in a closed container, and also could get metal to calcine in a closed container. The resultant gas from one of these processes (say the burning candle) could be tested in another (the mouse) and fail to support the new process as well, which gave him a warm fuzzy that all three gases were actually the same thing; air loaded to capacity with phlogiston.
Dephlogisticated air was prepareed by Scheele and Priestly separately but almost simultaneously in 1774. Scheele heated calx of mercury and collected the gas that came out; that gas would support combustion and respiration quite nicely so clearly it was air with no phlogiston in it at all.
Phlogiston was isolated by Henry Cavendish. This is the same Henry Cavendish who determined the value of the gravitational constant G over in physics land, as described in Part I of this series.
Cavendish added Mars (iron) to oil of vitriol to produce a gas which he collected in a bladder. The bladder actually floated in the air, which meant that he likely had phlogiston (which was supposed to have negative weight, after all), and the gas was also very combustible; logical for something released during burning. This new gas was “inflammable air” and had also been identified as being phlogiston.
So this looked very good for Stahl’s theory! Equations consistent with it could be written and phlogiston had indeed turned out to have negative weight.
We could even demonstrate that sulphur was oil of vitriol mixed with phlogiston, by use of those first two reactions I mentioned at the very beginning of this story.
By looking at all of this, it was clear that metals were compounds, and so was sulphur. The calxes and oil of vitriol were most likely pure substances, elements, irreducible to anything more simple.
Along comes Anoine Lavoisier. He made a fairly obvious prediction. Reacting phlogiston/inflammable air with dephlogisticated air should produce phlogisticated air.
It was already known that inflammable air was quite combustible, so Lavoisier built a very sturdy chamber for the reaction, one that would withstand the stress of the kaboom! and retain the product.
So then he did it, using a spark to touch off the reaction, and on examining the result he did not find phlogisticated air. Instead, he found the element water. And nothing else!
Think about that. There were other reactions that produced elements. But they always also produced something else. Starting with zinc and de-phlogisticated air, you could get the zinc calx element, but phlogisticated air would also be produced. In other words, if you start with a non element and turn it into an element, part of the original compound has to go somewhere else.
(zinc calx + phlogiston) + dephlogisticated air ->
zinc calx + phlogisticated air.
You can’t start with those sorts of beginning ingredient and end up with only an element afterwars. Whatever you broke away from the element has to have gone somewhere, in this case into the air to phlogisticate it.
So what’s going on here? How do you combine things and only get an element?
Fortunately, Lavoisier was a genius, and he did figure it out. By overturning every assumption that had been made.
He figured that water was a compound, a compound of inflammable air and dephlogisticated air. Up until this point water was presumed to be an element.
And that there was no such thing as phlogiston, and everything understood up to then was backwards.
If you understand modern chemistry at all, everything I’ve described up until now should seem inverted, like phlogiston is filling the role of oxygen, but in reverse–it is leaving things as they burn or rust, instead of combining with them.
But now, thanks to Lavoisier, try the new words “oxygen” for “dephlogisticated air” and “hydrogen” for “phlogiston” and “nitrogen” for “phlogisticated air.” These, Lavoisier realized are all elements; and air was a mixture of nitrogen and oxygen.
The metals weren’t compounds of something plus a “calx,” rather the calx was a compound of the metal and oxygen. And oil of vitriol was a compound of sulphur, not the other way around. (In fact today, oil of vitriol is called “sulphuric acid,” suitable for imbibing by your favorite Deep Stater.)
After several years of effort, Lavoisier was able to correctly identify 31 substances as elements, two still bear the names he gave to them (hydrogen and oxygen). Seven of these elements had not been isolated yet, but he figured they were part of a known compound; those are chlorine, fluorine, boron, calcium, magnesium, barium, and silicon.
Oddly he didn’t realize that potash and soda were similar; he thought they were compounds of ammonium. And he thought that heat and light were elements. (This was corrected by Count Rumford, who married Lavoisier’s widow.)
All in all, mistakes aside, this is a staggering amount of insight.
But he went further. In collaboration with three other chemists, he devised the naming system we use today. “Sodium chloride” is named according to this system; it indicates a compound of the two elements, sodium and chlorine. Gone was “flowers of zinc” to be replaced by “zinc oxide.” “Liver of sulphur” was now “potassium sulfide.” “Corrosive icy oil of tin” is now “stannic chloride.” And on and on, the new names reflecting the actual elemental composition. Most of the old names are now forgotten, but every once in a while you still hear them.
And now that elements were correctly identified, a lot of real progress could be made, because the whole mental map of what was going on was no longer upside-down and inside-out.
This is why Lavoisier is called “the Father of Chemistry.”
He was also a tax collector for Louis XVI. This made him well versed in accounting, which showed in his meticulous measuring of the masses of everything in reactions, to make sure the books balanced. He had demonstrated that mass was conserved in all chemical reactions.
Unfortunately his day job put his head into the guillotine in 1792 during the French Revolution. As Comte de Joseph-Louis Lagrange put it, “It required but a moment to cut off his head and perhaps a hundred years will not suffice to produce another like it.”
It took a long time for Lavoisier’s new chemistry to be accepted in Germany (the homeland of Stahl) and the United Kingdom was resistant as well. Politics had some influence on science back then too. But in England, it didn’t take too long. Because John Dalton would soon be hard at work, and so would Humphry Davy. These two parts happen almost simultaneously.
John Dalton
John Dalton made measurements of the masses of all reactants in many different reactions and came to the realization that elements reacted in certain fixed proportions by mass. (He managed this in spite of not being nearly as proficient at measurement as Lavoisier had been.) For example one unit of hydrogen appeared to react with 5.66 units of oxygen to form water. On the basis of this, he speculated that elements consisted of small minimum units, which he named atoms from Greek atomos, “can’t be cut.” This revived a speculation than had been dormant for over two thousand years, since Democritus who lived roughly around 400 BCE. He began publishing his work in 1806.
Dalton determined, very roughly, a lot of these ratios, and the ratios became what today are called “relative atomic masses.” The are the masses of atoms, relative to some (back then) unknown reference value. (In casual speech they are “atomic weights” and sometimes “atomic masses” though the latter can be confused with the actual mass of an atom in kilograms. Both “relative atomic mass” and “atomic weight” are officially sanctioned terms, though “atomic weight” seems to be falling out of favor. After all weight is actually a misnomer.)
Dalton carefully refined his table of atomic weights, but even his last effort is barely recognizable today. He had finally measured the oxygen:hydrogen ratio as 7, which was still not right, even given some of the bad assumptions he was making.
A lot of very basic (to us today) concepts were missing from this endeavour. It wasn’t clear that hydrogen and oxygen are never present as single atoms, but rather they’d form a compound with themselves, two hydrogen (or oxygen) atoms pairing off as a molecule of H2 or O2. Compounded atoms got the name “molecule.” This was true of nitrogen as well.
(On the subject of these diatomic elements, my high school chemistry teacher used to say that those elements whose names end in G, E, N or I, N, E were the “fags of the chemical world” because they’d form molecules with themselves. H2, O2, N2, F2, Cl2, Br2, I2 [for hydrogen, oxygen, nitrogen, fluorine, chlorine, bromine, and iodine, respectively]. I can guarantee you no high school teacher says that today. In any case, hydrogen has one bond, and shares it with the other hydrogen atom, oxygen has two bonds, and so is double bonded to the other oxygen atom in the molecule, nitrogen has three and triple-bonds. The “-ine” elements are all one bond each and are called, collectively, halogens.)
Also missing was the concept of valence; Dalton didn’t realize that it was possible for one atom to combine with more than one other atom, or even two or three times to the same other atom, and that different elements followed different rules in regards to this. Thus he never understood that water was H2O, not just HO. That caused him to understate oxygen’s atomic weight by a factor of two. He should have got oxygen = 8 on the basis of this misunderstanding, but he never quite got there.
All this emphasis I place on what he did not understand might lead you to think I am dumping on Dalton. No, absolutely not! Even with the things he didn’t know, he had made a huge conceptual leap, which (not incidentally) was needed before we could learn more. Ironically, the things he got right eventually made it possible for us to see his mistakes.
Amadeo Avogadro
Dalton’s misunderstanding of valence was corrected in part due to Amadeo Avogadro, who noted that when working with gases, their volume appeared to match these ratios. For instance a certain volume of hydrogen weighed two grams, matching its molecular weight; the same volume of oxygen would weigh 32 grams, matching O2‘s molecular weight. And when reacting, some volume of oxygen would combine with twice that volume of hydrogen to form water, in accordance with the H2O molecular formula for water, and not leave anything left over. Avogadro showed that at a given temperature and pressure, a certain volume of gas would contain the same number of molecules, regardless of which gas it was. Hydrogen, oxygen, Eric Swalwell’s most recent meal, it was all the same number of molecules per liter.
Today we know that 22.4 liters of gas at standard temperature (25 C) and pressure (1 atmosphere) will weigh, in grams, its molecular weight. That much H2 weighs two grams, that much oxygen, O2, weighs 32g.
Chemists found this useful, and defined a new concept, the “gram molecular weight.” Which got abbreviated “mole” and got the symbol mol. It’s now an official “base unit” of the modern International (Metric) System, alongside the second, the meter, the kilogram, and the ampere. (There are only two others, and you are about to meet one of those as well.) It’s basically the number of molecules it takes so that the numerical weight of the sample, in grams, is the same as its atomic weight. This is the same number for all pure substances, compounds or elements. We just didn’t know, then, what that number was, but that didn’t mean chemists couldn’t weigh out thirteen moles of copper sulfate when they wanted to.
Even though we didn’t know what the number was, or (equivalently) had no idea how much atoms and molecules actually weighed in grams or kilograms, Avogadro gets the credit for inventing the concept, and that number (now very well known today) is called Avogadro’s number in his honor and is symbolized by NA.
A good set of values for atomic weight became absolutely vital for chemistry. The unsung heroes of chemistry during the 1800s were those who put in years of exacting effort refining atomic weights. Their work wasn’t glamorous, and never would have won them Nobel prizes (if those had existed back then), but chemists knew these guys were doing something Very Important. The biggest “name” here was Jons Jakob Berzelius (who also discovered selenium and cerium oxide), who produced exceedingly good figures by 1826. And in fact people continue to refine the atomic weights, taking into account all sorts of factors we had no notion of until the 20th century.
It became apparent very quickly that atomic weights weren’t quite neat integers. It’s easy enough to quote that hydrogen’s atomic weight is one and oxygen’s is 16, but in fact both numbers are very, very slightly off from those integers, and this was not an artifact of inaccurate measurement. Rather, it’s the way things really are. This must have been maddening for chemists (Why be just a little way off from clean integer ratios? Why not a lot more off from them? It’s like mother nature was shooting at a target and just barely missed the bullseye. Why?)
A pause for an example of using moles.
Chemists making a compound could decide how many moles of it they wanted, for example, say, ten moles. Let’s say our goal is to start with hydrogen and oxygen and to produce ten moles of water. You start out with this idea of the equation for the reaction. It’s really a sort of shorthand recipe.
H2 + O2 -> H2O
Ten moles of H2O is going to contain ten moles of oxygen atoms, and twenty moles of hydrogen atoms, because there are two hydrogen atoms in every one water molecule.
But before you rush off and put 30 moles of gas into a container, there’s one thing to remember. The oxygen going into the reaction is not oxygen atoms, it’s oxygen molecules. And each of those contains two oxygen atoms. So you need five moles, not ten, of O2. And by the same token you need ten moles, not twenty, of H2.
So really, to include the quantities, we should write the equation like this:
10H2 + 5O2 -> 10H2O
But let’s sanity check it. Let’s see if mass is conserved.
Hydrogen’s atomic weight is one. Molecular hydrogen therefore has a molecular weight of 2. So ten moles of this is 20 grams of hydrogen.
Oxygen’s atomic weight is sixteen. Molecular oxygen therefore has a molecular weight of 32. So five moles of this is 160 grams of oxygen.
The total weight of all the ingrediens is 180 grams.
Over on the right hand side, the result is ten moles of water. Water, of course, has a molecular weight of eighteen (one + one + sixteen), and ten moles of it is therefore 180 grams.
The equation seems to balance.
Of course that equation only looks like it does because our goal was ten moles of water. To be generally useful it has to be reduced by dividing through by the lowest common factor. In this case that’s 5, so:
2H2 + O2 -> 2H2O
(One of the things taught in chemistry class is how to balance these equations, like we just did here. In some cases it can get very complicated.)
Any future chemist can scale this up or down, just like working with a recipe that doesn’t make enough (or makes too much) food for your needs.
Let me again emphasize that at this point we didn’t know the mass of any atoms and molecules, and therefore we didn’t know how many were in a mole. But it didn’t matter, we knew the ratios of those masses and could just use moles to keep those ratios consistent.
One last note about atomic weight before we move on.
Because oxygen reacts with a lot of things, and because (unless you are dealing with a gas) you pretty much have to be able to react with something to measure its atomic weight it was convenient to set oxygen’s atomic weight to exactly sixteen, and measure everything in terms of that. So hydrogen’s atomic weight was 1.008 (that’s the best number as of 1949). Much later on we ended up modifying this convention just a tiny bit.
More on Gases…and Heat
As mentioned, a mole of any gas will occupy 22.4 liters at standard temperature and pressure. What happens if you alter one of these parameters?
If you halve the volume, yet keep the temperature constant, you will double the pressure exerted by the gas.
On the other hand, if you double the temperature, either the volume will double and the pressure stays the same or vice versa.
…wait. FULL STOP.
What does it mean to double the temperature? If it’s 20° Celsius, is 40° Celsius twice as hot? Really? Well, 20° C is 68 F, and 40° C is 104 F. But 104 isn’t two times 68.
So it’s only twice as hot if you’re using a Celsius thermometer.
Well, that sure seems stupid, doesn’t it?
We don’t have this problem when doubling mass or halving a length or quadrupling an electric current or waiting for the end of the Biden administration, even if it seems six times longer than it is.
That’s because we can tell what zero mass (or length, or current) is. It’s pretty obvious; if you have none of something, its mass is 0 kg. So doubling the 5 in “5 kg” gives you “10 kg” and by golly, that really is twice as much.
The problem with temperature is that 0° F or 0° C isn’t really “no temperature” or “no heat” in any meaningful sense. What we need to do is to first realize that there’s actually a true zero point to temperature, then figure what it is. Then, it becomes possible to measure with respect to it.
We’re looking to determine absolute zero.
And it turns out we’re already on the right path. We don’t know what half or double the temperature is, but we can figure it out by cooling, or heating the gas until its pressure halves or doubles. And once we know that (just making up numbers) that 559° F is double the temperature of 50° F, we can backtrack and figure out what the real zero point is.
Chemists/physicists did something very much like this. They had to be careful not to let the gas liquefy (all bets are off if that happens), but it turns out that when they plotted the lines, an “ideal” gas would hit zero volume and pressure at -273.15° C, or about -459° F. This is absolute zero.
(I lied. I didn’t just make those numbers up. 50° F is 509° Fahrenheit degrees above absolute zero, so 509 + 50° F = 559° F is twice as hot.)
And chemists and physicists both use a temperature scale that starts at this point, with degree sizes the same as for Celsius (9/5 of a degree Fahrenheit). This is called the kelvin, after Lord Kelvin, an important figure in the history of thermodynamics. In fact it’s not even called “degrees kelvin,” it’s just “kelvins.” This is the sixth of the basic metric units.
300 K works out to 80.33° F, just to help you get a feel for it. And scientists consistently work in kelvins, everything from chemists having to figure out when a material will melt or boil, or how hot something must get before it will react, to astronomers telling you the temperature of Pluto, or Sirius.
As the 1800s wore on, it turned out that, deep down, the temperature of an object was directly related to the average kinetic energy of the molecules inside it. The total energy of the heat in the object is of course the sum of all the molecules’ kinetic energy, or in essence the total kinetic energy inside the object. But now we knew what heat was…it’s actually a manifestation of kinetic energy. And this is why when friction occurs objects heat up; the energy of motion is being transferred to the individual molecules. The object as a whole slows down, but the molecules start moving around with respect to each other (picking up the momentum the object loses, remember momentum is conserved) and the object heats up.
Humphry Davy
We now turn to the other thing that was going on starting in the 1800s (this time I don’t mean the century but rather the “zero years” of that first decade). I mentioned this in passing in part IV.
Sir Humphry Davy (1778-1829) exploited the voltaic pile (battery) to bust apart molecules that had been impervious to other methods (a typical method was to try to bring oxygen in to grab one constituent of a molecule, since oxygen is very good at “cutting in”).
The basic procedure was to prepare a solution of whatever it was you wanted to break apart, stick two electrodes into the solution, connect them to a battery, and wait for the electricity to do the work. One part of the molecule would collect around the positive electrode and the other part around the negative electrode.
Apparently, moving an electric charge around could induce at least some molecules to break apart.
Convinced that potash contained an undiscovered element (in spite of Lavoisier not thinking so), Davy made up a solution of it in water, hooked up the electrodes, and got hydrogen and oxygen. Whoops. He was busting up the water. But he needed a liquid for this to work. So he tried molten potash, and that worked like gangbusters. There were flames at the negative electrode. Taking a closer look, there were globules of silvery metal forming there, which would immediately burst into flame, just from contact with the air.
Davy was able to capture some of these globules before they self-torched and tried putting them in water. They’d race around the surface of the water and burst into lavender light. It turned out that the water was being broken apart into hydrogen and hydroxide (OH) and the hydroxide was reacting with the metal, to form KOH (potash lye). The hydrogen, on the other hand, was hot enough to spontaneously combust to form water vapor. Whatever this new stuff was, water would burn it!
According to witnesses, Davy danced around the laboratory with joy. He had just discovered potassium.
He tried soda (no, not coca cola). It took more voltage (electrical potential, the push) but he isolated sodium in short order. Sodium, of course is now famous for pyrotechnics when put into water. (It’s very, very dangerous, by the way, to simply throw a piece of sodium into a lake–a jet of hot, fresh soda lye (NaOH) might just shoot out the way the sodium came, land on you and blind you. However, I can promise Barry Obola that he is so anointed that he will come to no harm whatsoever if he does this. Trust me, Barry.)
Davy also nabbed magnesium, calcium, strontium and barium, elements that Lavoisier had identified as being there without them having been isolated. With the exception of magnesium, these would all spontaneously react with air and moisture energetically. Magnesium, the one metal that didn’t, was barely a successful find; it turned out a more successful method of isolating it was to react one of its compounds with pure sodium, so as it happens Davy was a key part of that effort anyway.
Davy had even more trouble with lithium; only small, wretchedly contaminated samples resulted from his efforts, and indeed it wasn’t until 1855 that good samples of lithium were isolated.
Michael Faraday (again)
All this was in 1807-1808, but Davy wasn’t done contributing to this story.
In 1813 he hired Michael Faraday. Yes, that Michael Faraday. The Michael Faraday, who alongside Newton and Maxwell, had his picture hung in Albert Einstein’s office. The Michael Faraday from last week that you were supposed to thank the next time you flipped a light switch (did you?).
Given that Faraday never had formal education, and learned all his science on the job, Davy did the world a tremendous favor giving him a chance. (So thank him, too, the next time you flip a light switch.)
As if unifying electricity and magnetism and laying the groundwork for modern civilization weren’t enough, Faraday also investigated electrolysis, following in Davy’s footsteps. In fact, he invented the words “anode,” “cathode,” “ion” and “electrode.”
Faraday is responsible for the discovery that in order to break a single bond, like say that between sodium and chlorine in salt, with electrolysis, a certain amount of electrical charge has to be supplied. And this number was the same per bond, per mol. This is, in fact, Faraday’s Constant.
To break one mole of single bond, it required 96,485.3 colombs. (Remember, once again, how humongous an electric charge one coulomb is.)
If it was a double bond, it would take twice as much charge.
This alone should be enough to convince anyone that there is a lot of electrical charge in simple, ordinary materials. We never noticed because it’s almost always perfectly balanced. When it falls out of balance, your sheets stick to each other coming out of the drier, your cat gets covered in packing peanuts, balloons pull your hair into a mess, and so on. On the plus side, if you can get the electrical fluid to move (without causing a huge imbalance) you can get it to work; a lot of work.
You could even think of this number as a mole of electric charge, since it operated to break one mol of single bonds (or half a mol of double bonds).
Chemistry, it was becoming quite apparent, is actually an electrical thing. Remember when I said, last time, that electricity is responsible for every physical phenomenon you see around you, except for gravity? That included things like why it’s hard to break rocks (electrical forces keep the rock bonded to itself), why water takes as much heat as it does to boil, anything having to do with light, and on and on. It includes things set on fire. It includes the question of why you and I aren’t just loose piles of disorganized atoms.
Dmitri Ivanovich Mendeleyev
(A quick linguistic note. Mendeleyev’s name is properly spelt: Дмитрий Иванович Менделеев, but I suspect most of my readers can’t read Cyrillic, so it’s necessary to transliterate his name into the Latin alphabet. Usually when this is done, the “y” is not included, but I think it’s better to use the y, because it is most definitely pronounced when English speakers pronounce his name (and for that matter is implicit in the second of the pair of еs in the original Russian). Those in the know know it’s “men-del-A-yev” rather than “men-del EVE” (it’s probably a way of hazing noob chemistry students who don’t know the trick and blunder) but the most-common transliteration doesn’t reflect this. Since the transliteration is supposed to be helpful, I decided to use the more-helpful, less-common alternative here.)
I started this article by pointing out that chemistry was a collection of unsorted trivia until Lavoisier, who finally got us on the right track to figuring out what substances were compounds, and which ones were elements, the basic building blocks of everything you can drop on your foot.
But Lavoisier knew of thirty one elements. By 1869 there were sixty three of them (including one mistake, didymium, that was really two elements that today we call praseodymium and neodymium).
This is an awful lot of different basic building blocks, isn’t it?
There seemed no rhyme or reason to it. Most of their masses were almost, but maddeningly not quite, integers, but even ignoring the tiny fractions, the numbers were chaotic. In order, hydrogen 1, lithium 7, beryllium 9.4, boron 11, carbon 12, nitrogen 14, oxygen 16, fluorine 19, sodium 23, magnesium 24 for the first ten.
What went into the holes? Was there something with an atomic weight of almost-but-not-quite 2, 3, 4, 5 or 6? What was up with beryllium?
Some chemists had begun to notice that some elements seemed chemically similar, for example, fluorine, chlorine and bromine, or copper, silver and gold, or chromium, molybdenum and tungsten. There seemed to be a lot of “triads” of elements like this.
But it was Dmitri Mendeleyev (1834-1907) who was the first to perceive the entire pattern…and to put a lot of confidence into it.
He sorted the elements according how they combined with oxygen. The first group (hydrogen, lithium, sodium, combined 2-1, two atoms of the element to one of oxygen. Each of these took up one of oxygen’s two bonds. You can write a generic formula, R2O for this. And to make the pattern clear, figure that an average atom of the first group combined with one half of an oxygen atom.
The second group was one-for-one. Beryllium, magnesium, calcium all took up both of oxygen’s bonds, generic formula RO.
Then there was a two-to-three group, boron, aluminum, etc, where two atoms of the element, with three bonds apiece, would combine with three atoms of oxygen, for a generic formula R2O3, or each atom combining with one and a half oxygen atoms.
This could be carried through until you got to elements that would combine with four full oxygen atoms (RO4), giving a total of eight possibilities, with elements sorted into eight groups.
Mendeleyev could sort these groups each by increasing atomic weight, then set these groups next to each other as columns in a grid. When he did that, he could read across, from group 1 to group 8, increasing atomic weights in the top row. Then the next row started in group 1 with a higher atomic weight and repeated the process. It was a periodic trend, every eighth element landed in the same group.
There were a few irregularities. For instance group eight, the one-to-four group, would either be empty on a given row, or hold three neighboring elements (iron-cobalt-nickel, ruthenium-rhodium-palladium, osmium-iridium-platinum), which was a bit of an irregularity, but it was a regular irregularity as every other row had one of these triples in column 8; the empty cells and the cells with three elements alternated.
That was far less interesting than some of the other irregularities in the sequence. For instance calcium belonged with beryllium and magnesium above it (and strontium and barium below it) in the one-to-one column, column 2. But the next element after that was titanium, which was a two-to-one which did not belong in the next three-to-two column which had boron and aluminum. Rather, it belonged better in column 4. So maybe this was all a waste of time?
Or maaaaybe the cell skipped over was a hitherto unknown element! So leave that spot open, and put titanium under carbon and silicon, the one to two column, where it belongs. (Titanium dioxide is a thing.)
There were two more holes between zinc and arsenic. And others, but Mendeleyev chose to focus on these three.
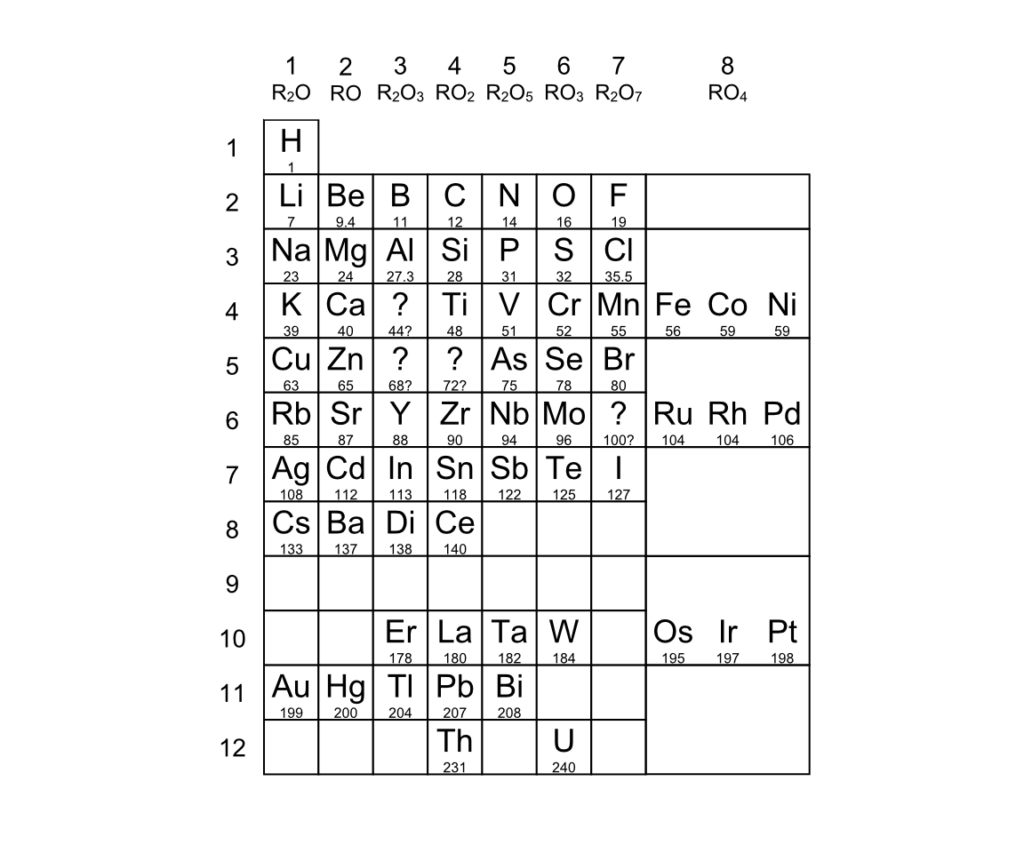
He wrongly placed Di, Ce, Er, and La (rows 8 and 10). Di (didymium) turned out to be two different elements,
but really La (lanthanum) should go in that square, not either of the two hiding in “didymium.”
Mendeleyev predicted three new elements to fill these holes. The first one he predicted an atomic weight of 44, an oxide R2O3 weighing about 3.5 grams per cubic centimeter. He made other predictions for the other two elements.
All three of these elements were found in the next 20 years, they are scandium, gallium, and germanium respectively. And they matched up with Mendeleev’s predictions pretty damn well. Not exactly, but far too close to be random chance.
Mendeleyev was definitely onto something. Previously, elements had popped up at random, with no rhyme or reason, totally unpredictably. A bright chemist might have a hunch that some mineral (say) had something new in it, and might even be able to prove it without isolating the element, but one could never tell when such a thing would turn up, or what the new element would be like, until isolated.
But now Mendeleyev could tell you, before anyone else had so much of an inkling as to the existence of an element, what it would be like!
Because of this, it didn’t take long for chemists to accept this pattern. It’s now called the periodic table of the elements. It has gone through several changes (the most important going from 8 columns to 18, or actually, 32) but it traces right back to Mendeleyev. It became so deeply ingrained, that chemists were even willing to disregard atomic weights if they were out of the periodic table sequence. In particular, 1889 a chemist named Brauner measured the atomic weight of tellurium very carefully and got a higher value than before, 127.6. This was a group 6 element, in the column headed by oxygen. Its next door neighbor in group 7 was iodine, and iodine had an atomic weight of 127. So now all of the sudden, tellurium had a higher weight than the next element in the sequence.
Does this mean that iodine and tellurium should swap places? Nope. Leave them where they are. There must be some reason for the oddity, but matching group membership was more important than arranging things in order by atomic weight. (Mendeleyev’s attitude was a bit different. He apparnetly figured the new number for tellurium must be mistaken; he wasn’t willing to part with the assumption that the atomic weights had to increase as you read across the rows, but he clearly did think the periodic sequence was more important; given a “contradiction” he went with the periodic table, not the atomic weight data.)
But even as the periodic table was being accepted as an organizing principle, it looked like it was starting to unravel. In the early 1800s chemists started discovering “rare earth elements” with atomic weights between 138 and 175. (No other elements were in this big gap.) They found more and more of these elements…and they were similar to each other, enough so that they were hard to separate, and the similarities were in fact why newer elements were able to hide within older ones. It’s like they were all trying to cram into the square below scandium and yttrium! (Mendeleyev knew of four in 1871, there would ultimately turn out to be fifteen of them.)
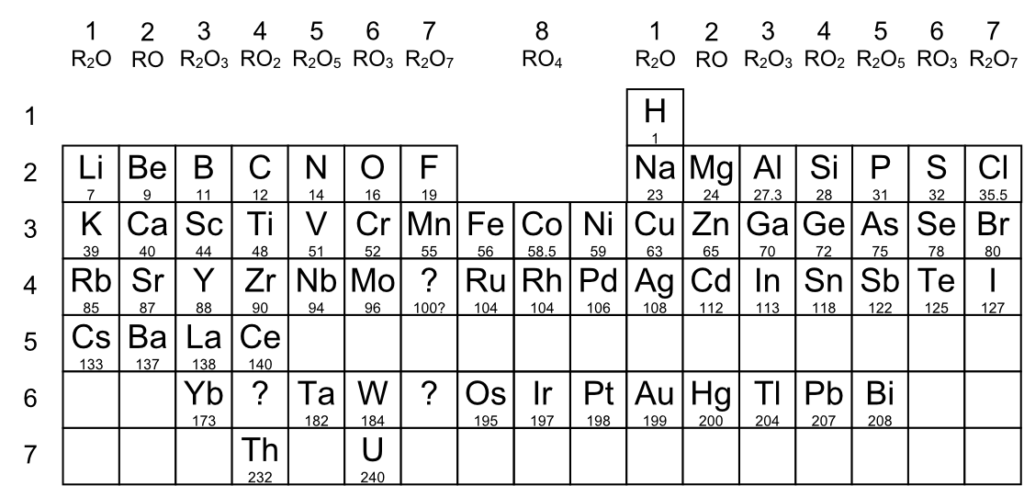
This is probably a bit more recognizable to modern eyes; rows 3 and 4 are almost dead-on as today’s rows 4 and 5.
The following rare earths are not included: Er (erbium), Tb (terbium), Ho (holmium), Tm (thulium,
Sm (samarium), Gd (gadolinium, Pr (praseodymium), Nd (neodymium), and Dy (dysprosium).
Nd and Pr are the two elements that had previously been combined as Di.
Ce and Yb are not on the right places (they are rare earth metals).
As more and more of these elements were discovered, Mendeleyev simply didn’t know what to do with them and just gave up trying to fit them in–leaving it for a future genius to solve. Other chemists tried to organize them and failed to do anything convincing with them. Since they didn’t follow the rules, there wasn’t even any way to know for certain how many of them there were!
So it was frustrating. There was only partial order to the elements, but then, where there was order, it was very, very useful. Call it a win, overall, even if it wasn’t a rout.
Sir William Ramsay
In fact, there wasn’t even any assurance that there wasn’t a totally unseen column in the table.
Wheatie asked me the question, once, as to whether there could be undiscovered elements between the ones we know about. Without pulling in a spoiler, the answer is basically “not no, but hell no!”
That’s the answer today, because of discoveries made in the 1910s. Back then, that was not the answer by a long shot; there were known holes in the table such as Mendeleyev’s three predictions. And who the heck knew how many of those damn rare earths there were, not to mention more holes like the one at atomic weight about-a-hundred.
Well, back up to 1785. Cavendish…remember him? G? Flammable air (i.e., phlogiston hydrogen)?
In a totally different experiment, Cavendish had reacted phlogisticated air (nitrogen) and dephlogisticated air (oxygen) with a spark, repeatedly, making niter. But some of the nitrogen just wouldn’t react. Since his source for these gases was the atmosphere, he was able to determine that this residue accounted for 1/120th of the atmosphere. (He was a very careful, meticulous and precise measurer, which is how he was able to determine G, a difficult thing to measure even today.)
And there that matter stood, basically forgotten, for almost a hundred years. Until 1882 when Lord Raleigh at Cambridge University’s Cavendish Laboratory (the irony!) was working with hydrogen, oxygen and nitrogen, trying to determine their densities and hence (thanks to Avogadro’s law) their atomic weights. He got good solid values for hydrogen and oxygen, but for nitrogen, he couldn’t get consistent results. If the nitrogen came from ammonia, his result was 1/2 of a percent lower than if the nitrogen came from the atmosphere. Raleigh was probably banging his head on the wall in frustration. He wrote to Nature, the preeminent scientific journal, asking if anyone else had any idea what was going on, just like today we might post on a chemistry forum online. He got a bunch of suggestions, including that the leftover gas might be N3, a hypothetical, less reactive form of nitrogen, just as oxygen could form O3 (ozone) instead of its usual O2.
Sir William Ramsay took another approach. He took some air, passed it over hot copper to remove the oxygen, hot magnesium to get rid of the nitrogen, soda lime to get rid of the carbon dioxide, and phosphorus pentoxide to get rid of the water vapor.
What he had left was about 1/80th of what he started with. At first he and others thought that this was indeed N3. But Sir William Crooks was able to prove that whatever this was, it wasn’t any kind of nitrogen.
In 1894, Ramsay realized the truth. This was a new element, one that didn’t react to oxygen at all. For that matter, it didn’t react with anything else either, including itself. This was argon, and it’s in every breath you take. An utterly non-reactive gas.
In addition to group 1, where every atom reacted with half an oxygen atom, through group 8, where every atom reacted with four oxygen atoms, in steps of half an oxygen atom, there was something one step to the left. Atoms that would react with no oxygen atoms.
This explained what Cavendish had seen.
There was a whole new column in the periodic table, call it Group 0.
Ramsay continued working into 1895 looking for other members of this column, unaware that he’d been partially scooped.
But 1895 is our line. We’re not quite yet ready to step across it.
Conclusion
There’s no new conservation law this time, rather a reinforcement of the conservation of mass and the conservation of energy, but we have plenty of mysteries.
Why are there so many different kinds of atoms? It’s nice that they form a pattern, but it’s not a perfect pattern, and those damnable rare earths really bork it in one place. Why is there a pattern, and why is it not perfect?
What is the relationship between atoms and electricity? We still don’t know what the electric fluid is. We have one tantalizing clue, that a bazillion coulombs (okay, 96,485.3 colombs, but that’s a lot) of charge seems able to bust up one mole of a single bonded molecule.
Remember, as far as we knew, an atom was an indivisible thing. Yet they seemed to be swapping electrical charges (or something) when forming compounds, with electrolysis somehow undoing that to break compounds apart.
All of which just pointed to a need to keep investigating atoms.
Obligatory PSAs and Reminders
China is Lower than Whale Shit
Remember Hong Kong!!!
中国是个混蛋 !!!
Zhōngguò shì gè hùndàn !!!
China is asshoe !!!
China is in the White House
Since Wednesday, January 20 at Noon EST, the bought-and-paid for His Fraudulency Joseph Biden has been in the White House. It’s as good as having China in the Oval Office.
Joe Biden is Asshoe
China is in the White House, because Joe Biden is in the White House, and Joe Biden is identically equal to China. China is Asshoe. Therefore, Joe Biden is Asshoe.
But of course the much more important thing to realize:
Joe Biden Didn’t Win
乔*拜登没赢 !!!
Qiáo Bài dēng méi yíng !!!
Joe Biden didn’t win !!!
