The above image from an old medical-scientific journal is from PeopleImages, via Google Images.
This post is part of Health Friday, a series of offerings related to Big Pharma, vaccines, general health, and associated topics. The discussion is not limited to what is presented today; it is an Open Thread. However, since this presentation is about a COVID-19 “vaccine”, the post is dedicated to the memory of Yours Truly’s cousin Bill, who died “suddenly and unexpectedly” in September 2023.
To begin, there are Important Wolf Moon Notifications, with a couple of extra items:
Free Speech is practiced here: “Use it or lose it.”
The following are alternate Q Tree sites for certain circumstances:
The U Tree is for “argue it out” interactions. There is a “Featured” article at this site for use as a “Rescue Thread.”
The “third site”, in case the above two are inaccessible.
Civil discussion is practiced here. The excellent and timely Rules of our late, good Wheatie prevail:
One: No food fights.
Two: No running with scissors.
Three: If you bring snacks, bring enough for everyone.
Please follow the added Guidelines from Wolf Moon. Please do not give the modern-day version of Cato the Elder the opportunity to show “enmity” to the board.
The extra items: What Yours Truly presents in this series, as in her other blog posts to this board, is not medical advice — the are opinions and hypotheses based on her over 4 1/2 years (and continuing) of reading about, researching out, and writing about “all things COVID”, Big Pharma, and other health topics. Readers are encouraged to consult a healthcare practitioner regarding health concerns or conditions.
The Health Friday post today concerns the impending Retraction of a peer-reviewed and published paper that details long-term COVID-19 “vaccine”-induced injuries in North India. The paper was submitted to Springer for review and publishing on 9 January 2024; it was accepted (it had passed the peer-review process); and it was published on 13 May 2024. Here is a free-access version (to read the entire paper on Springer, one has to either access through an institution, or to pay for a copy): www.qeios.com/read/JK7IBA/pdf, “Long-Term Safety Analysis of the BBV152 Coronavirus Vaccine in Adolescents and Adults: Findings from a 1-Year Prospective Study in North India”, Upinder Kaur, et al., 13 May 2024. The study was conducted at Banaras Hindu University in India. BBV152 is another name for the COVAXIN COVID-19 “vaccine”, developed by Bharat BIotech of India in cooperation with Indian Council on Medical Research (ICMR) – National Institute of Virology. ICMR receives “royalty payments” for each dose of COVAXIN that is administered, as does Bharat Biotech (Sound familiar? — as in, the co-development, co-ownership of patents, and sharing of “royalty payments” between the NIAID and Moderna for the modRNA COVID-19, mRNA-1273?)
For purposes of today’s offering, the trail begins here: https://blog.maryannedemasi.com/p/breaking-journal-pressured-to-retract, 23 September 2024. Dr. Demasi has a PhD in Rheumatology from the University of Adelaide (Australia.)
The Kaur, et al., paper referenced above was published by Springer on 13 May 2024. Almost immediately, the attacks began on the paper, the authors, and the publisher — with articles like this one: https://timesofindia.indiatimes.com/india/1-in-3-covaxin-recipients-hit-by-adverse-events-study/articleshow/110187284.cms, “1 in 3 Covaxin recipients hit by adverse events: Study”, 17 May 2024. On 18 May 2024, the ICMR demanded that Springer retract the paper (Yours Truly: nothing like causing panic in a government agency when the truth is published about a “vaccine” that the agency is pushing as “safe and effective,” especially when that agency is also getting “royalty payments” for the use of the “vaccine”, is there?). But, the attack on Springer and the authors didn’t end there. In July 2024, Bharat Biotech filed a lawsuit against Springer and the authors (some of the authors are students), demanding retraction of the paper and the payment of damages to Bharat Biotech of $600,000 US dollars (50 million Indian rupees.) In addition, the lawsuit accused the paper’s authors of defamation against the company, included with a demand for separate damages to be paid to the company for defamation. Despite sworn statements from the authors that no defamation was intended or written into the paper; and, despite the fact that Nitin Joshi, the editor of the Springer journal (Drug Safety) in which the study appeared, was one of the reviewers who approved the study for publishing, it was Joshi who notified the authors on 28 August 2024 that he was going to have the paper retracted. He confirmed this decision in an email to the authors on 17 September. However, as the defamation lawsuit is now in court, the study is still available on the internet.
What is BBV152/COVAXIN? It is an “inactivated whole virion vaccine” (whole virus vaccine) for “active immunization” against COVID-19. It is not an mRNA-based/modRNA-based COVID-19 “vaccine”, although it does use an “ancestral wave strain” of the original Wuhan Hu1 SARS-CoV-2 virus (in other words, a strain from the Wuhan Hu1 virus that occurred before the Beta, Delta, or Omicron strains.) The Package Insert for COVAXIN is here: www.bharatbiotech.com/images/covaxin/covaxin-pack-insert.pdf. Below is an image from the Package Insert:
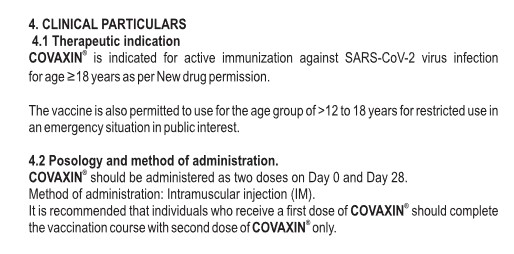
Per Wikipedia, 363,774,435 persons in India had been “vaccinated” with at least one dose of COVAXIN as of 4 March 2023.
However, the COVAXIN Package Insert does not actually describe how the “vaccine” works (the “Mechanism of Action.”) Yours Truly found something along the lines of the necessary information here: www.clinicaltrialsarena.com/projects/covaxin-bbv152-for-the-treatment-of-covid-19/?cf-view&cf-closed, “COVAXIN (BBV152) for the Treatment of Covid-19, India”, 28 June 2022. Below is a screenshot from this article:
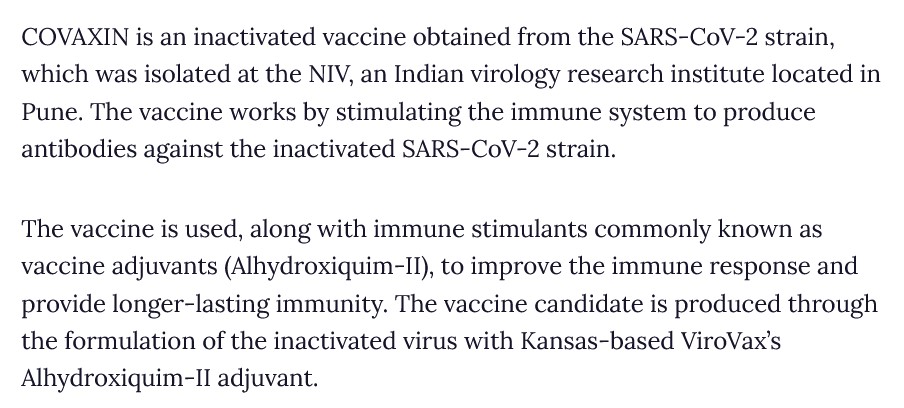
But, COVAXIN has been hailed by the Indian government about being “the first indigenous COVID-19 vaccine in India” (www.bharatbiotech.com/covaxin.html.) Why is ViroVax involved? (More on this later in the post.) Back to the Package Insert for COVAXIN. Below is a screenshot of the ingredients used in this “vaccine”:
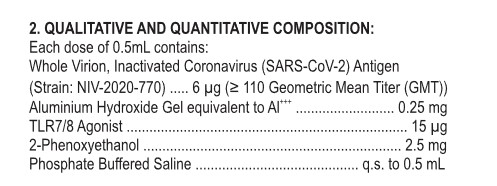
Looking further into the ingredients list, starting with the NIV-2020-770 strain of SARS-CoV-2: please refer to this paper: www.ncbi.nlm.nih.gov/pmc/articles/PMC7825810/, “Safety and immunogenicity of an inactivated SARS-CoV-2 vaccine, BBV152: a double-blind, randomised Phase 1 trial”, Krishna Mohan Vadrevu, et al., May 2021. It appears that NIV-2020-770 (the “inactivated whole virion”) is part of the Asp614Gly variant chain of the SARS-CoV-2 virus. The Asp614Gly variant itself is apparently part of the “ancestral wave” of the original Wuhan Hu1 SARS-CoV-2 virus; and, it is “not as serious” as the Beta or the Delta waves of the virus. Please refer to this article: www.thelancet.com/pdfs/journals/langlo/PIIS2214-109X(22)00199-1.pdf, Vol.10, July 2022, a Comment “Decoding the next SARS-CoV-2 variant”, by Jeremy Nel and WD Francois Venter. Below is a screenshot of a portion from the Comment:

Thus, BBV152/COVAXIN cannot be considered to be one of the “most recent” types of COVID-19 “vaccines”, as it does not include any elements before the Beta, Delta, or Omicron variants.
The Aluminium Hydroxide Gel in the ingredients (it is an “excipient” [“adjuvant]”): this is also called “Algel-IMDG” and “Alhydroxiqium-II” — in other words, it is a hydrogel. It was invented by ViroVax LLC of Lawrence, Kansas, under the aegis of the EpscoR Idea Foundation (part of the National Science Foundation in the United States), and with funding by the NIAID. This is the ViroVax / United States government connection. Here is a screenshot from an article by the EpscoR Idea Foundation on this “success story” (www.epscorideafoundation.org/success-stories/kansas-adjuvant-developed-with-nih-funding-enhances-efficacy-of-indias-covid-19-vaccine):
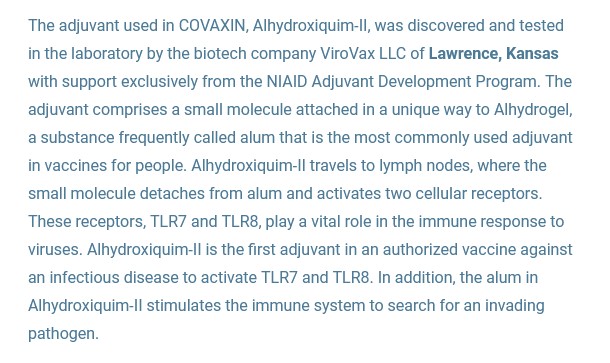
Note that Alhydroxiqium-II targets the lymph nodes of the person who takes COVAXIN. So, while this excipient is not exactly a lipid nanoparticle (LNP), it, in Yours Truly’s opinion, basically functions like an LNP in targeting the lymph nodes for delivery of the “vaccine payload” of COVAXIN; and, by “stimulating” the “vaccinated” person’s natural immune system to go and “search” for invading pathogens. By the way, Aluminium Hydroxide Gel is not to be used in foods, drugs pesticides, or “biocidal” products — see the screenshot below, from the search on Fisher Scientific (ThermoFisher) about this chemical, Page 1:

HOWEVER, it appears that FisherScientific had a “change of heart” since December 2021 regarding the “Uses advised against” for Aluminium Hydroxide — below is their MSDS Safety Sheet as of February 2024, Page 1:

Here is another source for an MSDS Safety Sheet, this one specifically for Aluminium Hydroxide Gel: www.oxfordlabfinechem.com/msds/ALUMINIUMHYDROXIDEGEL.pdf. Please see section 3 Hazards Identification; and section 11 Toxicological Information Special Remarks on Other Toxic Effects on Humans. Below is part of the Special Remarks portion of section 11:

The TLR7/8 excipient (adjuvant) in COVAXIN: This one is used in immunotherapy, including in the treatment of HIV-1. Please see: https://doi.org/10.3389/fmicb.2023.1033448, “Novel TLR7/8 agonists promote activation of HIV-1 latent reservoirs and human T and NK cells”, Yangyang Li, et al., 26 January 2023. This begs the question, Why is an HIV-1 immunotherapy treatment element being used in a COVID-19 “vaccine?” This also, in Yours Truly’s opinion, removes any consideration of COVAXIN to be labeled a “vaccine” — it is actually a gene therapy/immunotherapy injectable.
And, the 2-Phenoxyethanol excipient (adjuvant) in COVAXIN. Below is a screenshot portion of the Fisher Scientific (ThermoFisher) MSDS Safety Sheet for this chemical, Page 1:
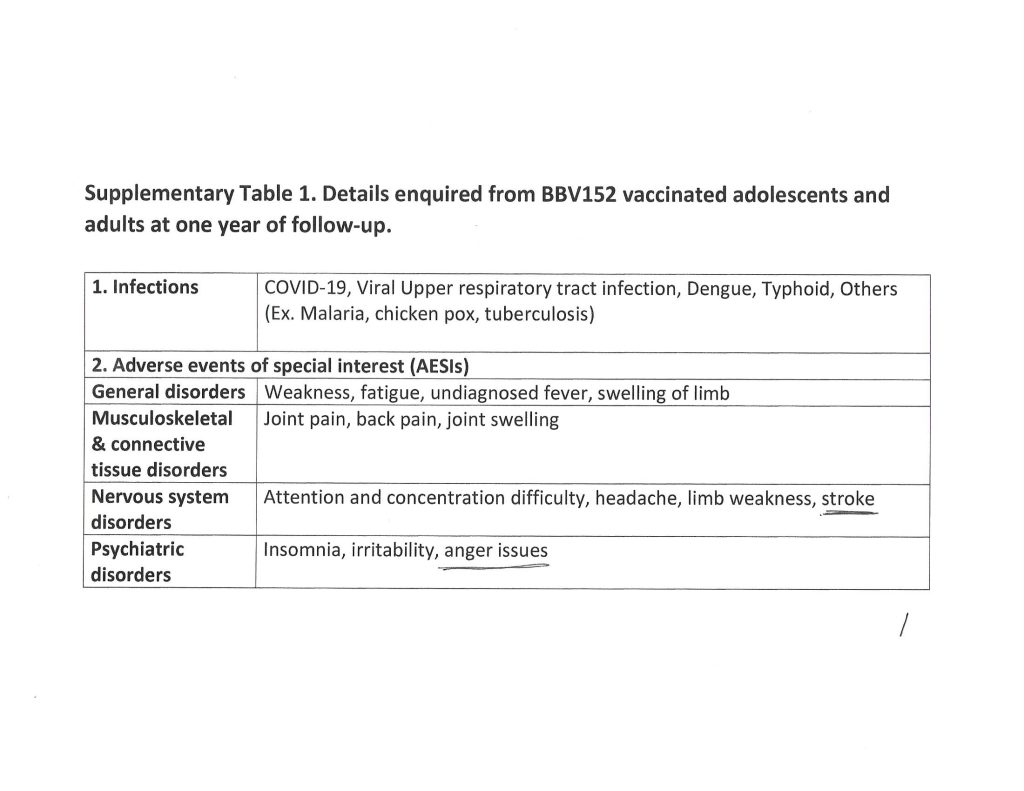
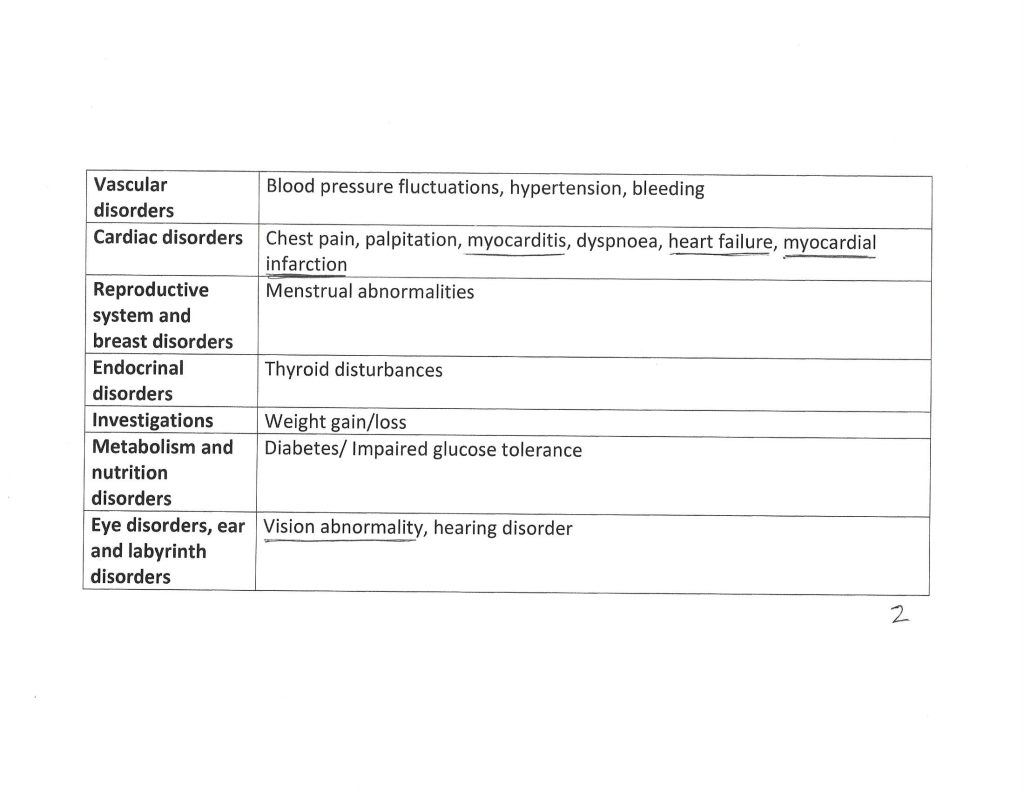
The Kaur, et al., paper, goes into detail regarding the types of reported serious adverse events that affected the study subject pool who took COVAXIN. Three pages of the Supplementary Table 1. from the paper are below. Note that these are details from subjects one year after “vaccination” with COVAXIN. Note also that another paper from December 2020 (by different authors) shows that the S1 protein of the SARS-CoV-2 virus itself crosses the Blood-Brain Barrier.
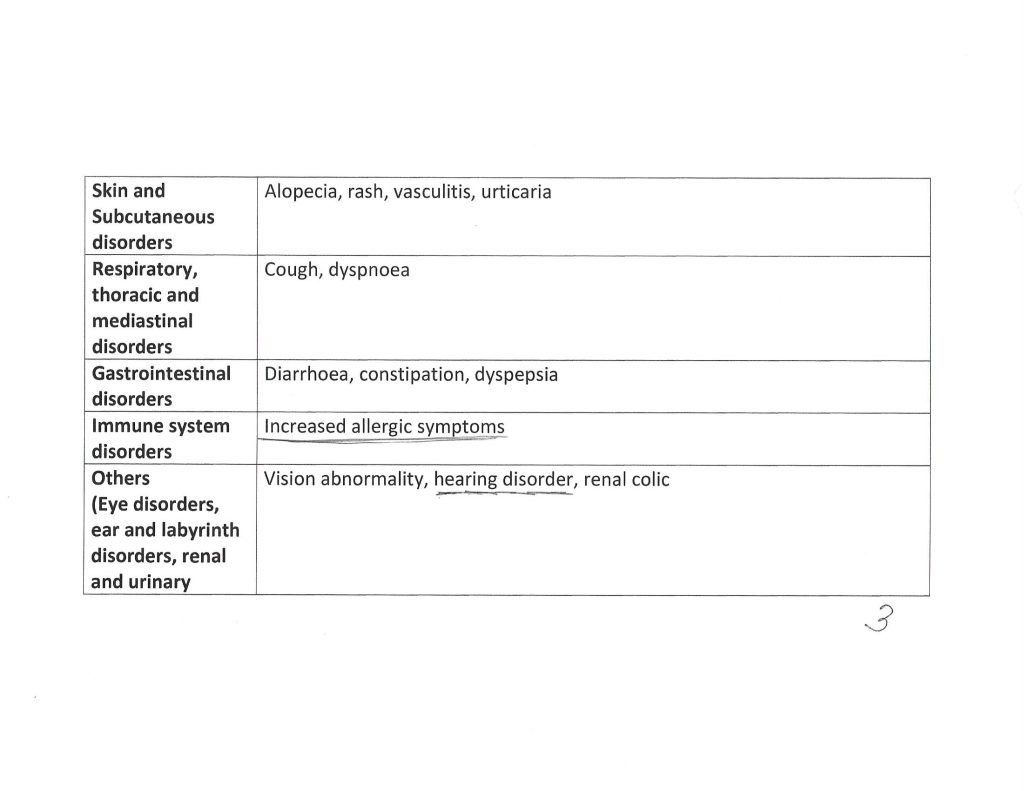
If Yours Truly is reading about this situation correctly, it appears that one of the “problems” with the Kaur, et al., paper, is that the subject pool of COVAXIN-“vaccinated” persons in North India who reported serious adverse events following “vaccination” to the study authors was “small.” One has to ask: How many COVAXIN-“vaccine”-induced serious adverse events would need to be reported before they would be considered “relevant” by the Indian government and by Bharat BioTech? — say, a “minimum” of 3 million adverse events reports? Is it “within acceptable limits” that COVAXIN-“vaccinated” persons in North India suffer a stroke or a heart attack after “vaccination” with this product? Is this another situation of “the known and potential benefits outweigh the known and potential risks” of taking a COVID-19 “vaccine”, which is the “official” position of the CDC and the FDA in the United States?
Yours Truly has gone into detail regarding the situation with BBV152/COVAXIN for several reasons: First, to enumerate the multiple potentials for “vaccine”-induced serious adverse effects from this COVID-19 “vaccine” product; Second, to highlight the persecution of the authors of a paper who sought to study and write about these potentials; Third, to highlight the persecution of the journal that peer-reviewed, approved, and published the paper; Fourth, to bring to light the involvement of the United States government (via the NSF and the NIAID) in the funding and development of an ingredient (excipient/adjuvant) in this “vaccine” intended for use in a foreign country; and, Fifth, to again emphasize how important it is that consumers “do their own due diligence” regarding information on drugs and/or injectables that they put into their bodies.
Peace, Good Energy, Respect: PAVACA