We should all remember Deplorable Patriot and Wheatie as we push forward with the fight. This is NOT over by any means.
Fight! Fight! Fight! Because JUSTICE must be served on those who foisted the “Vax” shit on us. And for all the other things they have done to this country.

You failed to pay attention to this advice.
You went out of your way to do the opposite.
You chose to rub our faces in it,
imprison those who dared complain,
and even to kill our people.
Now you shall pay just a tiny fraction of the real price, Ratfuckers.
What is it that feeds our battle, yet starves our victory?
RINO scum. Like Murkowski and Collins.
That’s OK. We go around ’em for now.
January 6 Tapes Reminder
OK…I’m sick and tired of reminding you to no effect, Speaker Johnson, so I’ll do the more emotionally satisfying thing and call you a cowardly, lying, fraudulent sack of diarrhetic monkey shit.
Johnson, you are a cowardly, lying, fraudulent sack of diarrhetic monkey shit!
A Caution
Just remember…we might replace the RINO candidates. (Or we might not. The record is mixed even though there is more MAGA than there used to be.) But that will make no difference in the long run if the party officials, basically the Rhonna McDaniels (or however that’s spelled–I suspect it’s RINO), don’t get replaced.
State party chairs, vice chairs, secretaries and so on, and the same at county levels, have huge influence on who ultimately gets nominated, and if these party wheelhorses are RINOs, they will work tirelessly to put their own pukey people on the ballot. In fact I’d not be surprised if some of our “MAGA” candidates are in fact, RINO plants, encouraged to run by the RINO party leadership when they realized that Lyn Cheney (and her ilk) were hopelessly compromised as effective candidates. The best way for them to deal with the opposition, of course, is to run it themselves.
Running good candidates is only HALF of the battle!
Justice Must Be Done.
The prior election must be acknowledged as fraudulent, and steps must be taken to prosecute the fraudsters and restore integrity to the system.
Lawyer Appeasement Section
OK now for the fine print.
This is the WQTH Daily Thread. You know the drill. There’s no Poltical correctness, but civility is a requirement. There are Important Guidelines, here, with an addendum on 20191110.
We have a new board – called The U Tree – where people can take each other to the woodshed without fear of censorship or moderation.
And remember Wheatie’s Rules:
1. No food fights
2. No running with scissors.
3. If you bring snacks, bring enough for everyone.
4. Zeroth rule of gun safety: Don’t let the government get your guns.
5. Rule one of gun safety: The gun is always loaded.
5a. If you actually want the gun to be loaded, like because you’re checking out a bump in the night, then it’s empty.
6. Rule two of gun safety: Never point the gun at anything you’re not willing to destroy.
7. Rule three: Keep your finger off the trigger until ready to fire.
8. Rule the fourth: Be sure of your target and what is behind it.
(Hmm a few extras seem to have crept in.)
Paper Spot Prices
All prices are Kitco Ask, 3PM MT Friday (at that time the markets close for the weekend). (Note: most media quotes are for the bid…the price paid by the market makers, not the ask, which is what they will sell at. I figure the ask is more relevant to people like us who wish we could afford to buy these things. In the case of gold the difference is usually about a dollar, for the PGMs the spread is much wider.)
Last Week:
Gold $3,085.20
Silver $34.17
Platinum $993.00
Palladium $994.00
Rhodium $6,275.00
FRNSI* 148.247-
Gold:Silver 90.290-
This week, markets closed at 3PM Mountain Time Friday for the weekend.
Gold $3,038.80
Silver $29.56 (Yikes!!)
Platinum $931.00
Palladium $943.00
Rhodium $5,875.00
FRNSI* 146.002-
Gold:Silver 102.801- (Again, Yikes!!!)
There’s no sugarcoating things. All of the metals except gold took a beating on Thursday. Then on Friday things got simpler. All of the metals took a beating.
At one point on Friday, gold was down over 90 bucks. As it is, by the end of the day it was down $77.90.
Gold was up over 3100 earlier this week and even crossed the magic $100/gram line (equivalent to $3110.35). I noticed on Thursday it had slipped below that line just a touch, looked at it Friday morning, read something ending in 20-ish dollars, and thought it had blooped up over the line again…then I realized it hadn’t gone up ten bucks, it had gone down ninety.
Silver took a harder hit. Note that the gold:silver ratio is now OVER A HUNDRED.
As a side note at least sometimes I title this section Paper Spot Prices (or something similar to that) as the spot price is ultimately derived from the commodities markets, which in turn trade paper gold and silver; futures that you’re expected to sell to cut your losses (or realize a profit). Since most people are in that market to make a buck, there are huge amounts of silver or gold contracts out there that will never actually be executed. This is always true. It’s when someone decides, “no I am taking delivery” that life gets entertaining; sometimes a LOT of people do that and then the person on the sell side of the contract is legally obligated to deliver. So more than likely he has to go out and buy 1000 ounces of silver, or 100 of gold. (Or 50 of platinum, when that market isn’t in a coma.) Suddenly, outside of the futures market there’s panic buying; people desperate to get their hands on the commodity they shorted; often paying much more than the buyer is going to pay them.
This can often lead to the market price for physical metal being quite different from the spot prices; a few years ago you simply couldn’t get gold for less than $200 over spot (and that was when it was much lower than it is even after today’s beating).
In the meantime, Silver is on sale right now folks!
*The SteveInCO Federal Reserve Note Suckage Index (FRNSI) is a measure of how much the dollar has inflated. It’s the ratio of the current price of gold, to the number of dollars an ounce of fine gold made up when the dollar was defined as 25.8 grains of 0.900 gold. That worked out to an ounce being $20.67+71/387 of a cent. (Note gold wasn’t worth this much back then, thus much gold was $20.67 71/387ths. It’s a subtle distinction. One ounce of gold wasn’t worth $20.67 back then, it was $20.67.) Once this ratio is computed, 1 is subtracted from it so that the number is zero when the dollar is at its proper value, indicating zero suckage.
Not Giving A F*ck
Kalbo (and then others) brought this to yesterday’s daily:
There are multiple ways to not give a f*ck. In this particular case Trump has decided he has a job to do, that 80 million Americans (at least) elected him to do that job, and if you don’t get out of his way you will be lucky if all that happens is you end up with his footprints all over you as he tramples you.
Flerfs Eat Their Own
Nothing like leaving a cult to get those left behind to pull out the long knives. And sometimes you don’t even have to leave, just be nearby when someone else does.
Mark Sargent (he’s probably the most famous Flat Earther to the general public; he’s the fairly clean-cut, blond guy with the baseball cap who gets interviewed a lot and showed up in documentaries) and Dave Weiss (Flat Earth Dave, the Potato, Dirth [his channel is DITRH], the guy with the leaky app), and two other prominent Flerfs who have not been named–have been sent “Cease and Desist” letters by lawyers for three ex-Flerfs for claims the flerfs have made about them. (Text visible at approximately the 5:50 mark). One of ex-Flerfs is Patricia Steeres, who was Mark Sargent’s co-host until recently, then she left. (Mark has characterized it as a “breakup” even though they never dated.) The other two are Robby Davidson and “Paul on the Plane.” (These two are not ones I am familiar with except I think Robby Davidson is known for having quit Flat Earth as soon as he realized Dave Weiss and Eric Dubay had no interest in going to Antarctica in spite of saying so earlier. Too obviously they were bluffing and their bluff had been called.)
Did they cease and desist? Well, no. MC Toon did a livestream over 4 1/2 hours demonstrating that Sargent, at least, did not do so. I’m going to link it but I certainly don’t expect you to watch it unless you are an absolute glutton for punishment:
[Another fun activity on these long MC Toon livestreams is he has people sign into the chat and try to warn people that Dirth’s app is leaky, just to see how fast their comments get censored and themselves get banned. Clearly Menagerie is in their employ. He will also call the Flerfs up and leave taunting voicemails when they don’t answer.]
Next…some Flerfs are going after Lisbeth Acosta. Lisbeth is the Flerf who won a free trip to Antarctica, which turned out to be a sham prize. Will Duffy was suckered into awarding it and then the donor turned out to be a Flerf troll. There was an INSTANT rallying of globers to contribute to pay for her ticket so she got to go anyway. Apparently what she saw did not convince her, though since she decided to be Mark Sargent’s co host when Patricia Steeres left. (McToon begged her not to take the job.)
Sticking with Flat Earth isn’t enough though, since Fkatzoid decided to go after her.
Apparently, Lisbeth was prostituted out to the other Final Experiment goers to get them to toe the Globe Earth line when they came back. Fkatzoid calls her the “Village Bicycle.” This too is worthy of a lawsuit, however Fkatzoid lives in South Africa and has no money. (His job is mixing paints.) Perhaps some of the others can be gone after.
So not only is this guy the absolute best evidence for the Dunning-Kruger effect that I have ever seen (remember he argued against Critical Think’s weight experiment, and also go into it with Will Duffy about the location of the south pole), he is an absolutely shitty individual who would deserve a throat punch and a curb stomp even if he wasn’t an idiot.
Isochron Dating
Recall from last time that uranium-lead dating done on zircons lets one assume there were no daughter lead isotopes in the zircons when the zircons were first formed. That’s because the zircon crystallization process rejects lead while accepting uranium. However, there’s always the possibility that after some period some of the daughter isotopes (the lead) will leach out of the zircon crystals, which will have the effect of making the dating result look younger than it actually is.
The fact that there are two different pairs of uranium-lead parent-daughter isotopes allows us not only to detect that that has happened, but to correct for it, by taking several samples out of the same igneous rocks and then plotting the results on a “concordia diagram” then drawing a straight line to intercept the curve plotted for ideal cases where no lead has been lost.
Zircons can often turn out to be much older than the rocks they are in; they melt at a very high temperature and granitic magma doesn’t typically get that hot. So if you find a zircon in an igneous rock, it might be much older than that rock.
So to use uranium-lead dating in other places (not zircon crystals) we need a way to account for the likelihood that there was lead present in the rock when it formed. Then uranium lead dating can be used in more situations. And we can use it for other sequences, for example the rubidium-strontium decay (rubidium-87 to strontium-87 by beta decay, half life 49,720 million years; rubidium is element 37, strontium is element 38) and the samarium-neodymium decay (samarium-147 to neodymium-143 by alpha decay, half life 106,000 million years; samarium is element 62, neodymium is element 60). (There is another isotope of samarium, Sm-146, that has a half life of 92 million years, decaying by alpha decay to Nd-142, which could conceivably be used, however, that half life is just short enough that we can no longer detect any natural traces of samarium-146…so that clock has run out.)
All three sequences–four, really since there are two uranium-lead sequences–can benefit from isochron dating. (Isochron comes from the Greek for “same time.”) They aren’t the only ones, but they seem to be mentioned most often when I find an article about isochron dating.
Isochron dating is done by taking multiple samples. It works so long as: the samples all have the same origin (minerals from the same rock, rocks from the same geological unit)–this ensures that all samples had the same initial isotopic composition. And we assume nothing leaks out of the rock over time (the opposite of the situation with the zircon crystals, which could lose lead over time).
Note that there is no assumption that the daughter isotope was absent from the rock initially.
One more thing that is needed, is a non-radiogenic isotope of the daughter element. For rubidium-strontium strontium-86 fits the bill; nothing decays into that isotope. And for samarium-neodymium, neodymium-144 is used. Again nothing decays into it. (However, it is very slightly radioactive with a half life of 2,290,000,000 million years, about 170,000 times the age of the universe. Not enough to matter; in fact so little of it has decayed so far we can’t even think of using it for dating in a hypothetical neodymium-cerium dating sequence; we’d get no reading at all.)
Let me put that into a handy-dandy table:
Method
Rb-87->Sr-87
Sm-147->Nd-143
U-238->Pb-206
Half-life (My)
49,720
106,000
4,468
Non-radiogenic or reference isotope
strontium-86
neodymium-144
lead-204
Rubidium and strontium are admittedly obscure to the man in the street, but they are workaday elements, appearing to some extent in many rocks. Rubidium is potassium’s big brother, somewhat rare but it will substitute for potassium in minerals. Strontium, similarly is calcium’s bigger brother. Calcium is very common in the Earth’s crust, and strontium atoms will occasionally substitute for them. These elements are stable, or thought of as being stable, but as it happens 27.8 percent of all rubidium is actually rubidium-87, so your typical sample of rubidium is actually weakly radioactive. The daughter strontium-87 isotope is 7 percent of all strontium, while the reference isotope Sr-86 is 9.86 percent of all strontium. (Almost all the rest of the strontium is Sr-88.)
Samarium and neodymium are rare earth elements…yes, actually rare earths. They tend to be dispersed throughout the crust and there are few ores. Nevertheless, “rare” is a bit of misnomer; on average there is about three times as much samarium in the crust as there is tin. 15 percent of all samarium is samarium-147 (which means that samarium-147 by itself is roughly half as common as tin), but with a 106 billion year half life, you can probably think of it as just barely radioactive. The decay product, Nd-143, is roughly 12.2 percent of all neodymium, and the reference isotope, Nd-144, is 23.8 percent of all neodymium (and is very, very, very weakly radioactive).
So yes these isotopes can be found in rocks, fairly readily.
How Isochrons Work
Recall from last time we showed formulae expressing radioactive decay just showing the simple case where we started out with no daughter isotope. Here is a slightly more complex formula for the number of daughter isotope atoms:

This one has a D0 term, which is the initial concentration of daughter isotope atoms; i.e., what was in the rock when it formed. n is the present number of parent isotope atoms. The entire second term is the number of daughter isotope atoms that have resulted from the decay of the parent isotope, from the formation of the rock to the present day. Note that this formula is written in terms of the decay constant, not the half life. See the prior post for more information on this, but it’s 1 divided by [the half life multiplied by the natural logarithm of 2].
Since the isotopes are measured by mass spectrometry, it’s more convenient to deal with the ratios between the numbers, not the absolute numbers. So here is where we introduce the reference isotope (the non-radiogenic one); we’re going to divide all terms by that number, to get a bunch of isotope ratios.

The first term is the total amount of daughter isotope, divided by the total amount of the reference (non-radiogenic) isotope. This is something we measure. The second term is the initial amount of daughter isotope, divided by the amount of reference isotope. We don’t know this, because we don’t know the initial amount of daughter isotope. (But note, we’re not claiming this number is zero, as we were with the zircons.) The third parentheses surround the amount of parent isotope today, divided by the amount of reference isotope. This is something we can measure. The final bit is the proportion of daughter isotope generated by decay (so far) of the parent isotope; which depends on the age, which we don’t know.
But this is very very similar to:
y = b + xm
…which is the “generic” equation for a line (albeit rearranged a bit). b is where the line crosses the y axis, and m is the slope of the line. So if we substitute as follows:
y = D*/Dref (we measure this)
b = D0/Dref (we don’t know this but it’s constant for a given rock)
x = Pt/Dref (we measure this)
m = eλt – 1 (we don’t know this but it’s constant for a given rock)
…well we might be able to do something about this. Note that in the line equation, b and m are supposed to be constants. Indeed for a specific rock, of some age (which we don’t know yet), D0/Dref (b) is indeed a constant; it should be the same everywhere within the rock. As should eλt – 1 because every part of a given rock is the same age, this is m. Of course m is the slope of our straight line. Note that it gets steeper the higher t goes.
The two things that correspond to x and y are the things we actually measure. So we can plot our measured y against our measured x and now we have one point on this line. Well by itself one point isn’t useful. We expect m will be a positive number, and b will be above zero (since there is more than zero daughter isotope in the rock)
So take another sample, of a different mineral in the same rock. Then take a few more. Plot them, y versus x.
If all of those points fall on a straight line…we can draw the line and figure out m and b. The first will tell us how old the rock is (by solving for t), the second is actually going to tell you how much daughter isotope there was initially; information that might be interesting but doesn’t directly help us date the rock.
If the line is not straight, something probably happened to the rock after it formed, that invalidates our assumptions. If you have six points and only one is out of line, you can treat it as an outlier (but of course when you write up your paper, you point this out!).
Examples
Here’s a sample (apparently not a “live” sample but just an illustration). Note that different minerals from the same rock are all analyzed, as well as “whole rock”
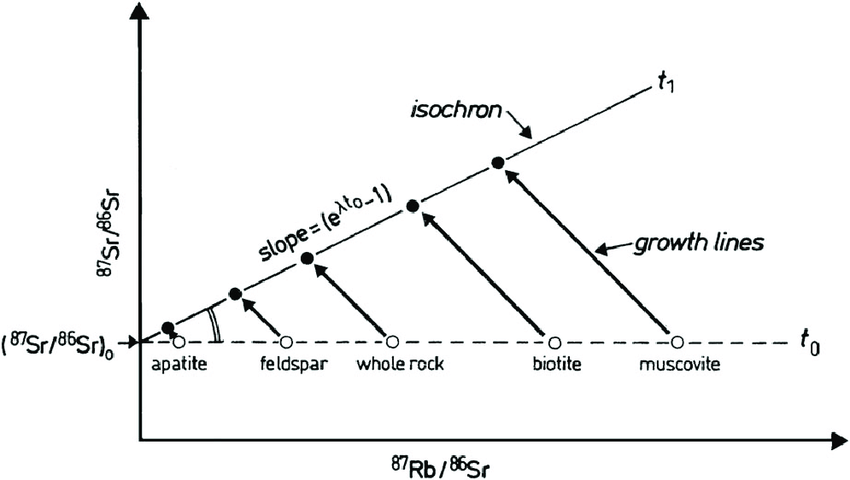
The X axis is the present day parent (rubidium 87) – reference (strontium-86) ratio (matching what I showed above as being “x”), and the Y axis is the daughter (strontium 87) to reference (strontium-86) isotope ratio. The y intercept is labeled as being the initial daughter/reference ratio; that tells us how much daughter isotope there was originally. And the slope is our decay term, the steeper the slope, the higher the value of t is.
Here’s an actual plot from a real measurement. Note that three of the minerals tested are clustered very close together near the left hand margin, and the computed ratio of daughter isotope present at the beginning, to the reference isotope, is 70 percent. And finally notice the age: 609.5 million years (give or take 2.5 million years).
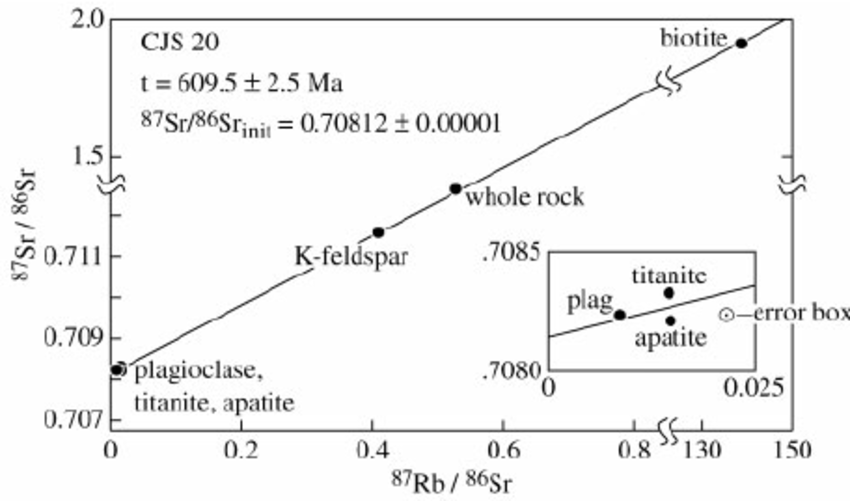
So the short version of this is, isochrons can help you identify and correct for the sorts of things that those with a little bit of knowledge of radiometric dating might bring up as objections, of the form “but what if there was some daughter isotope already present?” But it will only work if the rock hasn’t lost any daughter isotope since it was formed; if it has, the line won’t be straight. The good news is when this happens, the data says it happened, and if you’re alert you won’t be fooled.
A bit more of this (I want to cover potassium-argon dating in particular, and then discuss carbon-14 dating even though it’salmost totally irrelevant to geology) and we’ll get back to the main narrative.
