The above word-cloud image about memory loss is courtesy of Google Images.
This post is part of Health Friday, a series devoted to Big Pharma, vaccines, general health, and associated topics. Today’s offering, a Special Edition, is in honor of Yours Truly’s “fully vaccinated and boosted” brother, who was just diagnosed with “sudden-onset dementia.” Yours Truly will make it clear that one is not a medical doctor; one is not treating my brother; and, this post is a “narrative primer” on negative neurological effects from the COVID-19 “vaccines”, the Pfizer-BioNTech COMIRNATY (BNT162b2) modRNA COVID-19 “vaccine” in particular.
There are Important Wolf Moon Notifications; the Rules of our late, good Wheatie; and, certain extra items that readers should be familiar with. They are all linked here.
For purposes of today’s post, the trail begins here: https://mole.substack.com/p/who-monkey-pox-is-side-effect-of-covid/comments, “WHO: Monkeypox Is ‘Side Effect’ of Covid ‘Vaccine'”, 12 October 2024.
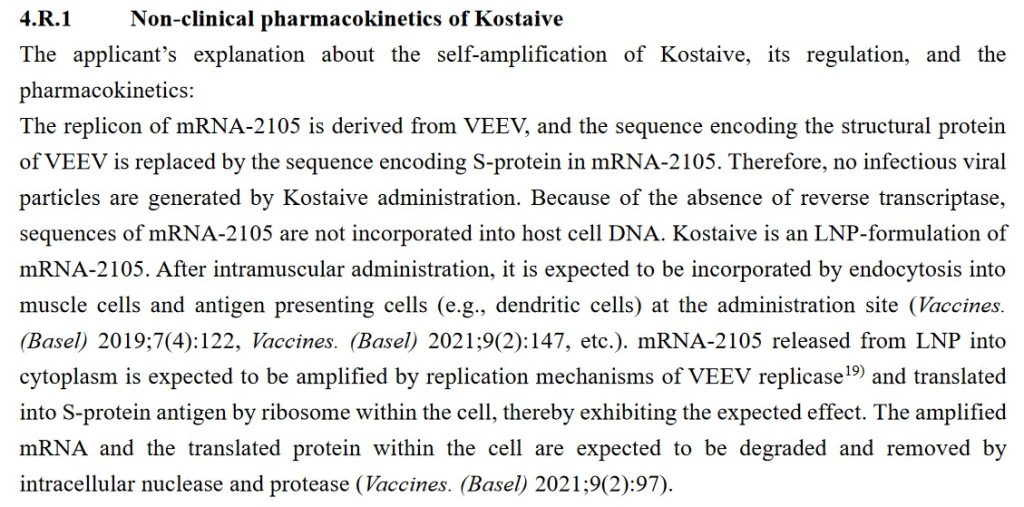
The World Health Organization (WHO) has a website, www.vigiaccess.org/, that lists “side effects” reported in persons who took COVID-19 “vaccines.” The Mole article describes how the VigiAccess search was performed to find “Monkeypox” as a “side effect” of the Pfizer-BioNTech COVID-19 “vaccine”, BNT162b2. A screenshot of the search protocol is below:

Yours Truly performed a VigiAccess search for reported memory problems (dementia is considered to be a form of “memory impairment.”) This is what I did:
Went to www.vigiaccess.com/
Clicked on “Search database”
Typed “BioNTech” into the Search box
Clicked on “Pfizer BioNTech COVID-19 Vaccine” on the list that came up
Clicked “OK” on the “Dialog” box
Under “Reported potential side effects”, I clicked on “Nervous system disorders” — and found a long list. A screenshot of a portion of this list shows “Memory impairment”:

These are only the reported cases of conditions that are considered to be “side effects” of BNT162b2. It can be argued that there are many multiples more “side effects” cases from BNT162b2 that are not reported for some reason. Yours Truly also performed searches on the VigiAccess list under “Psychiatric disorders”, and under “Metabolism and nutrition disorders”, as certain other details were given to one in phone conversations related to the situation which raised interest.
The balance of today’s post will be presented in a “quasi-scientific paper” form. With the exceptions of some in-line references, scientific paper, blog and/or article citations will be numbered in the text with [“number”], and listed at the end of the post. Hypotheses and opinions of Yours Truly (H/O) will be delineated by Bold text with Italics. A General Summary will be included at the end of the post.
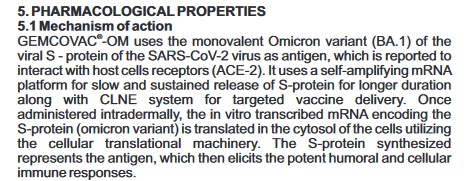
First: A Short Narrative Summary of What Occurs When a Person is Injected with the Pfizer-BioNTech modRNA “Vaccine” COMIRNATY: The “vaccine” here is the 2024-2025 Formula COMIRNATY COVID-19 “vaccine” (which, by the way, contains elements of BNT162b2, the original Pfizer-BioNTech modRNA COVID-19 “vaccine.”) Each “vaccine” dose is either supplied in a single-dose vial, with the dose to be administered withdrawn for injection; or, is in a pre-filled syringe ready for administration. (H/O) Upon “vaccination”, the person’s body immediately sends an “enemy detected” signal to the brain. The person’s body may react from fainting to chills to nausea, among other physical responses: see Sections 5.1 and 5.2 of the COMIRNATY 2024-2025 Fact Sheet, below [1]:

The lipid nanoparticles in the “vaccine” quickly begin to spread the contents of the injection throughout the “vaccinated” person’s body. This process is called biodistribution. Images of page 7 and page 8 of the January 2021 Pfizer-BioNTech Pharmacokinetics Tabulated Summary of the company’s modRNA COVID-19 “vaccine”, BNT162b2, are below [2]. Yours Truly will again emphasize that BNT162b2 is the basis for all of the Pfizer-BioNTech modRNA COVID-19 “vaccines”, including the COMIRNATY 2024-2025 Formula.
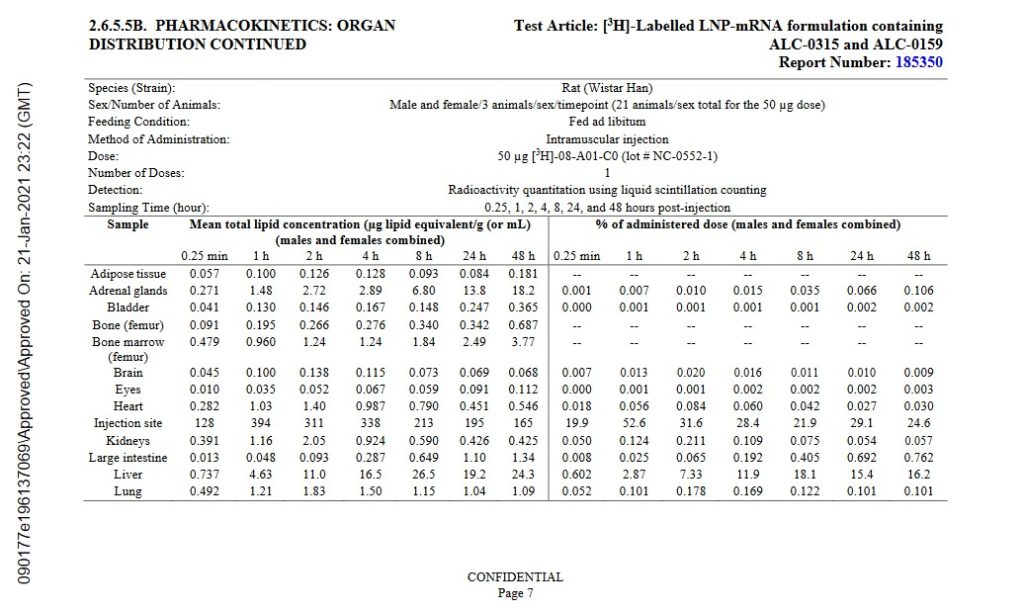
Note the accumulations in the Brain, the Liver, and the Large Intestine.
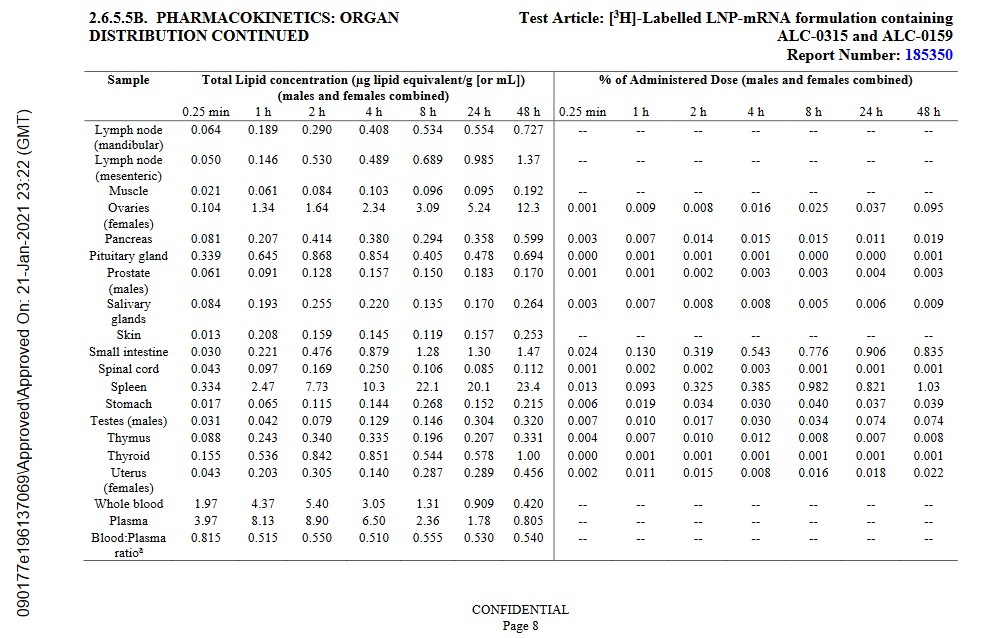
Note the accumulations in the Pituitary Gland, the Thymus Gland, and the Small Intestine. The intestines produce 90% of the body’s Serotonin. Serotonin is a neurotransmitter that is involved in the emotional / psychological / cognitive processes of the brain (per Wikipedia.)
Why is there accumulation of BNT162b2 in the Pituitary Gland? This small organ, called the “master gland” of the body, produces or directs many important hormones. Together with the hypothalamus, the pituitary gland work together to serve as the “brain’s central command center to control vital bodily functions”, according to Yours Truly’s online search at the Cleveland Clinic. These functions include breathing, stress response, reproduction, blood pressure, and more. (H/O): BNT162b2 and/or COMIRNATY is a “vaccine” to supposedly “prevent” a COVID-19 virus infection, not to work like a sort of pituitary gland treatment vehicle.
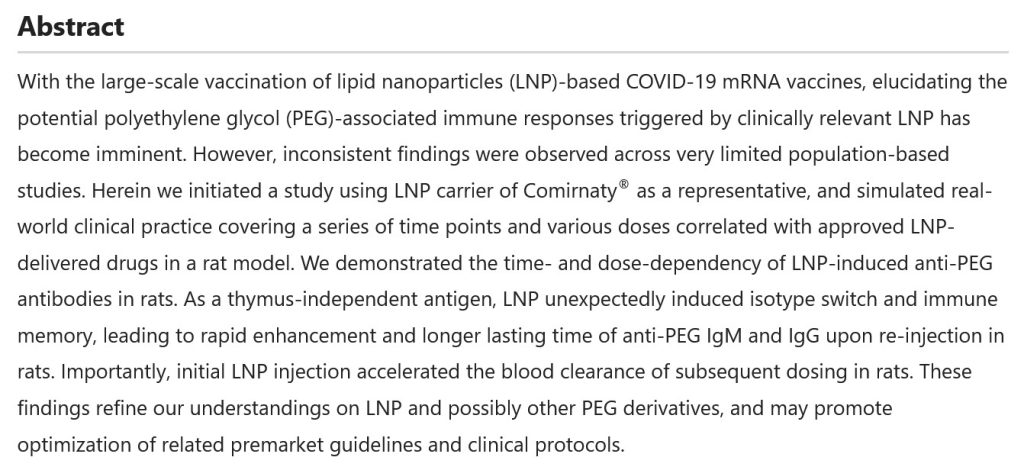
At least one of the lipid nanoparticles (LNPs) in COMIRNATY, ALC-0159 (but listed under its chemical component name), affects the thymus gland of the “vaccinated” person’s body. The thymus gland is “the primary lymphoid organ of the immune system” (per Wikipedia). The Abstract of a paper [3] that explains how ALC-0159 and other PEG-based LNPs work in the body is below:
While the lipid nanoparticles (LNPs) in COMIRNATY are spreading the ingredients of this “vaccine” throughout the “vaccinated” person’s body, the “vaccine” is using the PRRARSV “backdoor key” (an element which is only in the SARS-CoV-2 virus; and therefore, by extension, the modRNA COVID-19 “vaccines”) to facilitate entry of the “vaccine” ingredients into every cell in the “vaccinated” person’s body [4},[5]. (H/O) At the same time, the ingredients and mechanisms of COMIRNATY (BNT162b2) are beginning to work on inducing accelerated aging in the “vaccinated” person’s body, all the way down to the mitochondrial level [6]. Dao-Fu Dai, et al., wrote a paper in 2014 on how oxidative stress affects the mitochondria and the aging process [7].
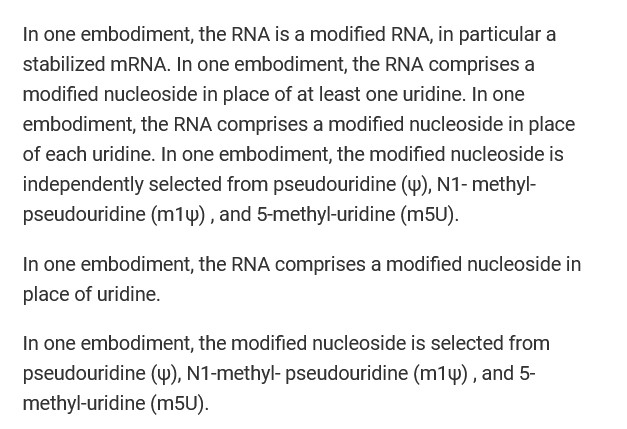
N1-Methylpseudouridine is present in all versions of COMIRNATY (BNT162b2), starting with the “original version” of this “vaccine”, BNT162b2. N1-Methylpseudouridine is a synthetic form of Uridine. Uridine is an important nucleoside for neurological processes, for the central nervous system, and other body processes. It is found only in RNA. Below is the Abstract of the Yueyuan Yang, et al., paper that the mechanism of Uridine in the body [8]:
The importance of Uridine cannot be minimized. This RNA element is produced in the Liver. Uridine helps to regulate mood; it also assists in the release of dopamine in the brain. Dopamine is a neurotransmitter that “affects emotions, behavior, and movement” (per WebMD.)
The purpose of N1-Methylpseudouridine in COMIRNATY is twofold: One, to “supersede” / “overwrite” the mechanisms of the natural Uridine in the “vaccinated” person’s body; and, Two, to enhance the mechanism of COMIRNATY in “mRNA switches in cells” of the “vaccinated” person’s body (Callum JC Parr, et al.) [9]. Below is the Abstract of this paper:

Yours Truly will blow the lid off the deliberate inclusion of N1-Methylpseudouridine in BNT162b2 (COMIRNATY.) This chemical was specifically added to the “vaccine” to replace the processes of natural Uridine; and, to evade the body’s natural immune system’s “enemy detection and elimination” elements and mechanisms. Below are screenshots from the Global Patent for BNT162b2, 28 October 2021 [10}:
Note that this appears to be a tacit admission that Gain-of-Function experiments were performed to create the modRNA in BNT162b2.

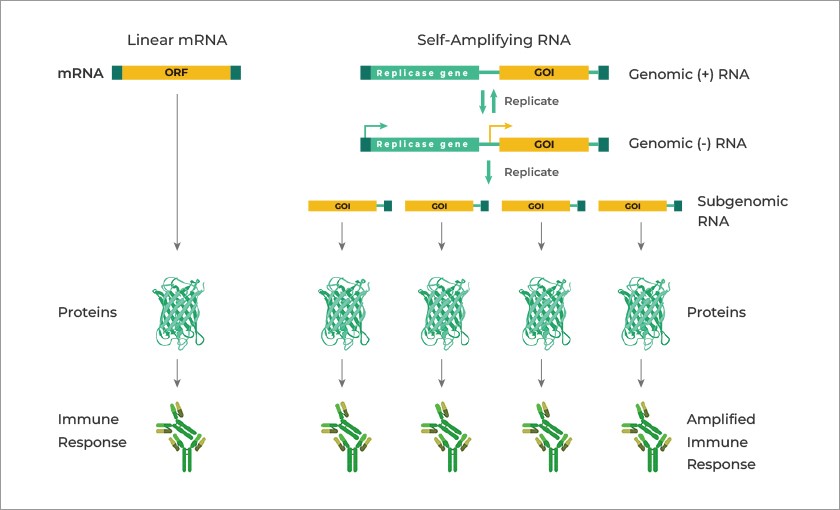
Note that this appears to be an admission that self-amplifying RNA (saRNA) can be used in Pfizer-BioNTech COVID-19 “vaccines.”
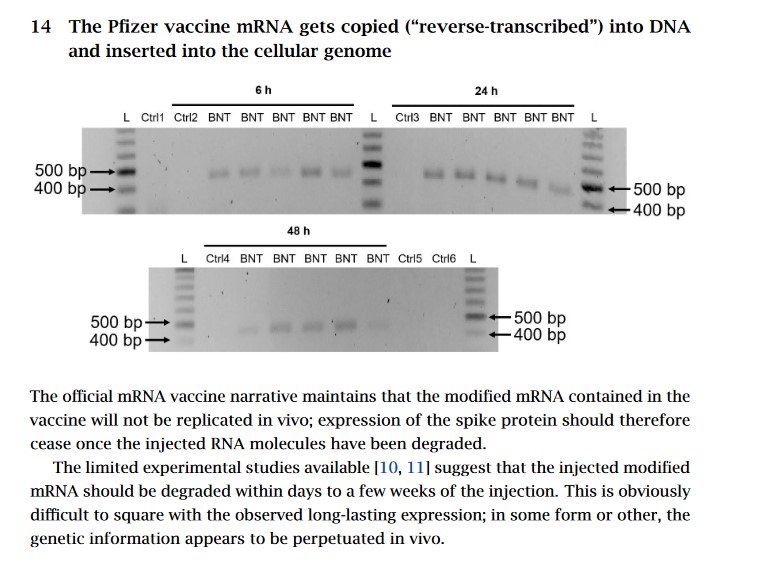
In addition to the “replacing” of uridine with N1-Methylspeudouridine in the body of the person “vaccinated” with COMIRNATY, the DNA of that person’s body is also being changed; please see Slide 14 of this article for a graphic of how the modRNA COVID-19 “vaccines” change the DNA.
Meanwhile, the newly-COMIRNATY-“vaccinated” person’s body, having “detected” an “enemy within the gates” (the “vaccine”), is sending out “all-out effort” signals to the body’s natural immune system to manufacture large amounts of cells to “fight off” the “detected enemy.” These natural immune system cells include CD4 cells, CD8 cells, IgG3 cells, and lymphocytes. However, the ingredients and mechanisms of the “vaccine” hamper, damage, and/literally destroy these natural immune system cells, via the “class switch” [11]; while, at the same time, inducing oxidative stress and cell death at the mitochondrial level all over within the “vaccinated” person’s body; and, also, inducing a state (of a still-unknown timeframe) of “faux SARS-CoV-2 infection” in the “vaccinated” person’s body [12]. A screenshot from this University of Maryland Medical System is below; note the last sentence:

The “rationale” behind the development and use of the modRNA COVID-19 “vaccines” is that the human body is not capable, of itself, to detect and “fight off” an infection by the SARS-CoV-2 virus; the body must have “assistance” in the form of the modRNA COVID-19 “vaccines” and their mechanisms. (H/O) However, it can be fairly argued that, given the multiple Adverse Events reports regarding COMIRNATY to VAERS and to VigiAccess, that this “vaccines” needs much more thorough R&D processes, testing on lab animals and then on humans, and extensive data collection and analyses, before this “rationale” can be fully proven. Pfizer-BioNTech rushed BNT162b2 into production, into securing the initial Emergency Use Authorization from the FDA in December 2020, and into this modRNA COVID-19 “vaccine” being used on the general public, before any of the above were fully undertaken. Pfizer-BioNTech and the FDA knew, back in April 2021, that BNT62b2 could, and did, cause hundreds of serious Adverse Events [13].
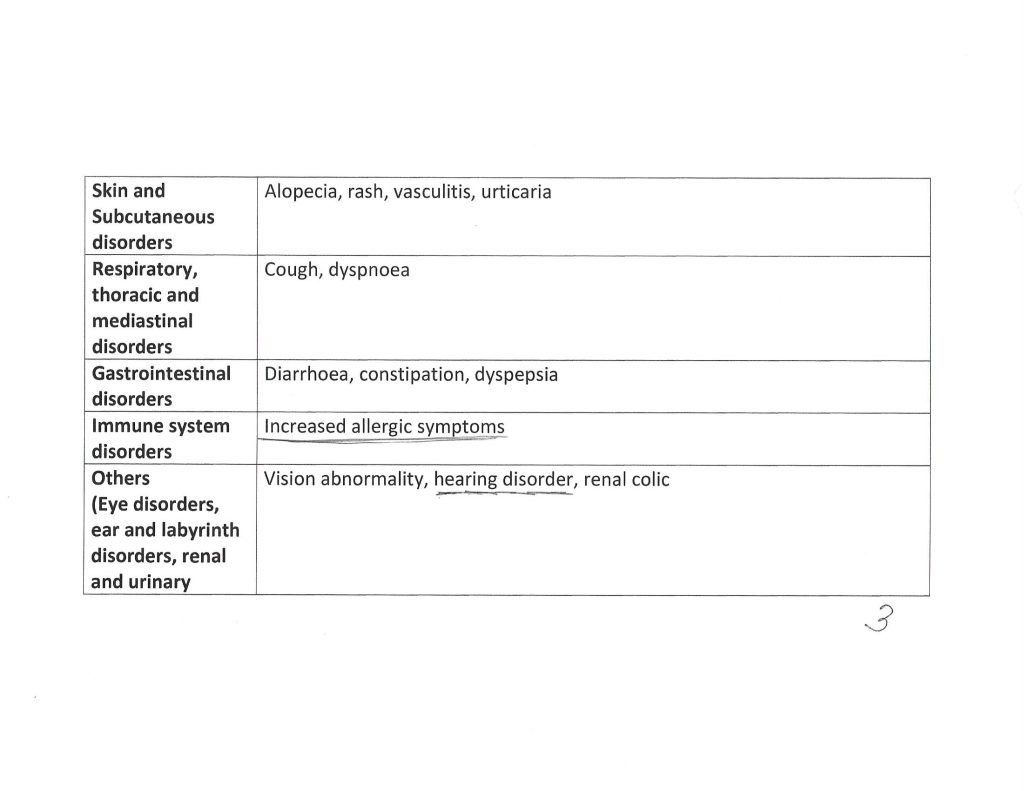
Second: Physical Neurological Effects of the COVID-19 “Vaccines”: These negative effects are somewhat “easier” to identify, as they can present with unmistakable symptoms. From the Appendix 1. List of Adverse Events of Special Interest in the 5.3.6 Cumulative Analysis document on BNT162b2 that was cited above, some examples: Page 2: Brain stem embolism; Brain stem mycoplasmal (encephalitis); Central nervous system lupus; Cerebellar artery thrombosis; Cerebral venous thrombosis. Page 3: Demyelination; Embolic stroke; Encephalitis autoimmune; Encephalitis post-immunisation. Page 4: Epilepsy; Epileptic psychosis; Grey matter heterotopia; Guillain-Barre syndrome. Page 5: IIIrd nerve paralysis; Immune-mediated encephalitis; IVth nerve paresis. Page 6: Meningitis; Multiple sclerosis; Neuritis; Neuromyelitis optica spectrum disorder; Neuropsychiatric lupus. Page 9: Thrombotic stroke; XIth nerve paralysis.
Bell’s Palsy is also reported after BNT162b2 “vaccination” [14].
In other words, COMIRNATY (and BNT162b2) damage multiple mechanisms and processes of the brain that result in physical medical conditions and/or illnesses. This “vaccine” damages the sheath coverings of the nerves, including of the spinal cord (Demyelination.) This “vaccine” causes brain inflammations of various types (Encephalitis.) This “vaccine” causes stroke. This “vaccine” causes nerve paralysis. And more. (H/O) This “vaccine” can also potentially aggravate existing physical neurological conditions and/or illnesses, including ones that were under control before the patient was COVID-19 vaccinated.”
There is also what may be considered a “hybrid” condition, since it involves both physical and psychological symptoms, and since can be brought on by either an infection by the COVID-19 virus itself, or by COVID-19 “vaccination”: Long COVID (also called Long Vax.) Long COVID appears to be an “overraction of the immune system” [15]. Physical symptoms include blood pressure swings, fatigue, and “brain fog.” Psychological symptoms include depression, anxiety, and even PTSD [16]. The FLCCC Alliance has articles on Long COVID / Long Vax.
Third: Psychological Effects of the COVID-19 “Vaccines”: The Blood-Brain Barrier (BBB) consists of “closely-spaced cells” that act as a protective barrier to keep many substances from reaching the brain. Below is the National Cancer Institute definition of the Blood-Brain Barrier [17]:

However, COMIRNATY (and the BNT162b2 before it) were specifically developed to cross the Blood-Brain Barrier and to damage glial cells of the brain, down to the mitochondrial level [18]. A screenshot of section 4. Conclusions of this paper is below:
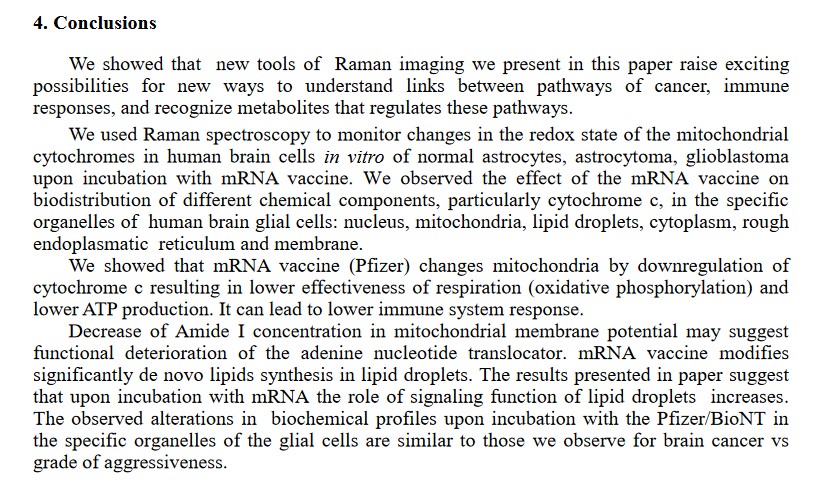
Recall that COMIRNATY (and the BNT162b2 before it) contain the lipid nanoparticle, ALC-0159. This lipid nanoparticle, one of the four within the “vaccine”, assists in the spread of the “vaccine” to all areas of the “vaccinated” person’s body, including into the brain.
Does the crossing of the Blood-Brain Barrier by COMIRNATY (and the BNT162b2 before it) also affect the psychological processes of the brain? The answer is, Yes: and, most likely, via the use of the N1-Methylpseudouridine in this “vaccine.” Recall that natural uridine, (which is one of the nucleosides that make up the RNA of the body) is produced by the liver. Uridine influences the brain by assisting in regulating mood, behavior, movement, and more. N1-Methylpseudouridine was deliberately included in COMIRNATY (and the BNT162b2 before it) to replace the natural uridine in the “vaccinated” person’s body with a created “faux uridine” (see the screenshots from the BNT162b2 Global Patent document, above in the post.) This chemical was chosen because it was “more effective” than the other two chemicals listed in the Global Patent documentation screenshot above in the post.
The Roh, et al., paper of 28 May 2024 traced a potential association between COVID-19 “vaccination” and the onset of Alzheimer’s disease (AD) and also of Mild Cognitive Impairment (MCI) [19]. A screenshot from this paper is below:
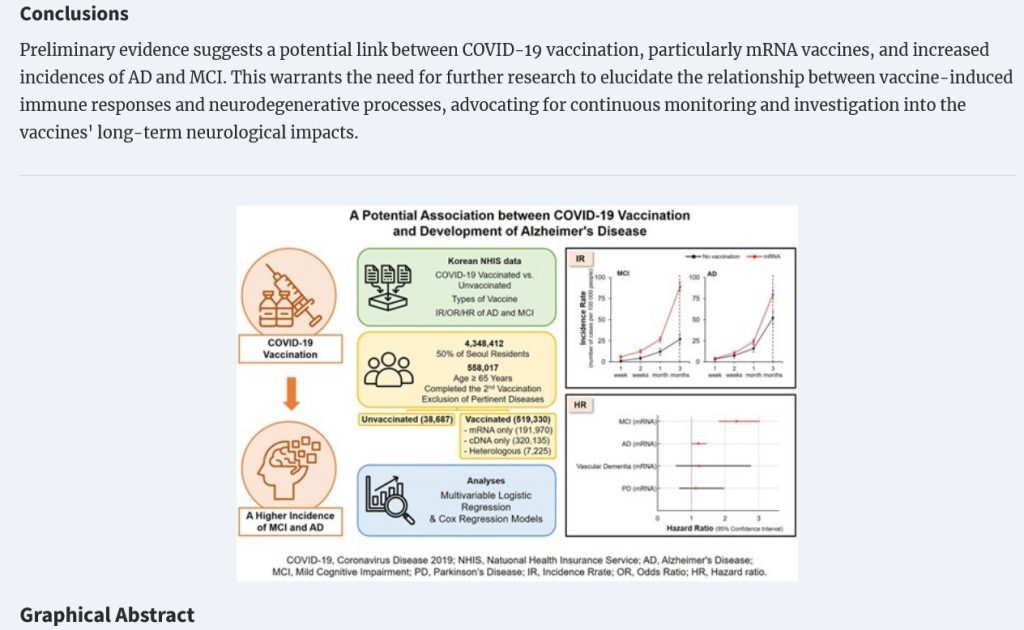
While there is not yet a proven association between MCI and the onset of dementia, the potential is higher for onset of dementia in persons who have MCI [20].
At the same time, there is documented proof that COVID-19 “vaccination” can cause psychosis. Examples of papers published on this topic are: the “Aljeshi paper” [21]; the “Borodina, et al., paper” [22];, the “Lazarevna, et al.” paper [23]; the “Morz paper” [24]; and, the “Laxmi and Grover paper” (psychosis diagnosed months after COVID-19 “vaccination” [25].
Yours Truly will again emphasize that one is not a medical doctor; nor am I treating my brother. (H/O) However, I believe there is sufficient evidence to at least consider the possibility that COMIRNATY (BNT162b2) modRNA COVID-19 “vaccinated” persons are at risk of numerous negative effects to the brain, both physical and psychological, induced by this “vaccine”; AND, since it is unknown exactly how long the elements and mechanisms of COMIRNATY (BNT162b2) work in the “vaccinated” person’s body, the possibility also exists that the potential for any physical or psychological neurological negative effects from this “vaccine” may actually increase with additional injections of COMIRNATY (BNT162b2.)
General Summary: One: The Pfizer-BioNTech modRNA COVID-19 “vaccine” COMIRNATY (and, the company’s modRNA COVID-19 “vaccine” BNT162b2 before it) can induce multiple negative physical and psychological neurological side effects and/or Adverse Events conditions in persons who take these “vaccines.” Two: COMIRNATY (and BNT162b2) contain dangerous lipid nanoparticles, among them, ALC-0159, which quickly spread the ingredients of this “vaccine” throughout the “vaccinated” person’s body, including to the brain, the intestines, and the pituitary gland. Three: COMIRNATY (and BNT162b2) contain the lab-created chemical, N1-Methylpseudouridine, which replaces the natural Uridine in the “vaccinated” person’s RNA. Four: Pfizer-BioNTech included N1-Methylpseudouridine in the formulation COMIRNATY (and BNT162b2) and stated this in the company’s Global Patent documentation for this “vaccine.” Five: Pfizer-BioNTech tacitly admitted that Gain-of-Function experiments were performed in the development of BNT162b2, per the Global Patent documentation for this “vaccine.” Six: Pfizer-BioNTech admitted that self-amplifying RNA (saRNA) can be used in COVID-19 “vaccines”, per the Global Patent documentation for this “vaccine.”
In addition, the following may be of interest: One: A blog article by Alex Swanson, M.S., on uridine, 30 November 2020; and, Two: The FLCCC protocol on recovering from COVID-19 “vaccination.”
The list of citations follows. Peace, Good Energy, Respect: PAVACA
[1] www.fda.gov/media/151707/download, Full Prescribing Information for COMIRNATYR (COVID-19 Vaccine, mRNA) suspension for injection, for intramuscular use 2024-2025 Formula
[2] https://icandecide.org/wp-content/uploads/2022/03/125742_S1_M2_26_pharmkin-tabulated-summary.pdf, BNT162b2 2.6.5 Pharmacokinetics Tabulated Summary, FDA-time stamped 21 January 2021
[3] https://doi.org/10.1038/s41541-023-00788-z, Polyethylene glycol (PEG)-associated immune responses triggered by clinically relevant lipid nanoparticles in rats, Haiyang Wang, et al., 2 Nov. 2023
[5] https://doi.org/10.1101/2022.09.27.509633, Nuclear translocation of spike mRNA and protein is a novel pathogenic feature of SARS-CoV-2, Sarah Sattar, et al., 27 Sept. 2022
[7] https://doi.org/10.1186/2046-2395-3-6, Mitochondrial oxidative stress in aging and healthspan, Dao-Fu Dai, et al., 2014
[8] www.ncbi.nlm.nih.gov/pmc/articles/PMC10937367/, Uridine and its role in metabolic diseases, tumors, and neurodegenerative diseases, Yueyuan Yang, et al., 29 Feb. 2024
[9] https://pubmed.ncbi.nlm.nih.gov/32090264/, N1-Methylpseudouridine substitution enhances the performance of synthetic mRNA switches in cells, Callum JC Parr, et al., 6 Apr. 2020
[10] https://patents.google.com/WO2021213945A1/en, CORONAVIRUS VACCINE Global Patent document, 28 Oct. 2021
[11] https://jessicar.substack.com/p/igg4-cd4s-and-why-the-lnp-mrna-platform, IgG4s, CD4s and why the LNP/mRNA platform should be prohibited, 14 Aug. 2023
[12] www.umms.org/coronavirus/covid-vaccine/mrna, Understanding the COVID Vaccine and mRNA, 30 Sept. 2022 (NOTE: This link no longer works as of 17 October 2024. There is a new link: www.umms.org/health-services/covid-19/about-the-vaccines.)
[13] www.phmpt.org/wp-content/uploads/2022/04/reissue_5.3.6-postmarketing-experience.pdf, BNT162b2 5.3.6 Cumulative Analysis of Post-authorization Adverse Event Reports; please see Appendix 1. List of Adverse Events of Special Interest. FDA time stamped 30 Apr. 2021
[14] https://doi.org/10.1016/S1473-3099(21)00451-5, Bell’s palsy following vaccination with mRNA (BNT162b2) and inactivated (CoronaVac) SARS-CoV-2 vaccines: a case series and nested case-control study, Eric Yuk Fai Wan, PhD, et al. 16 Aug. 2021
[15] www.science.org/content/article/rare-link-between-coronavirus-vaccines-and-long-covid-illness-starts-gain-acceptance, Gretchen Vogel and Jennifer Couzin-Frankel, 3 July 2023
[17] www.cancer.gov/publications/dictionaries/cancer-terms/def/blood-brain-barrier
[18] https://doi.org/10.1101/2022.03.02.482639, Decoding COVID-19 mRNA Vaccine Immunometabolism in Central Nervous System: human brain normal glial and glioma cells by Raman imaging, H. Abramczyk, et al., 2 Mar. 2022
[19] https://doi.org/10.1093/qjmed/hcae103, A potential association between COVID-19 vaccination and development of alzheimer’s disease, Jee Hoon Roh, et al., 28 May 2024
[20] www.alz.org/alzheimers-dementia/what-is-dementia/related_conditions/mild-cognitive-impairment
[21] www.psychiatrist.com/pcc/psychosis-associated-covid-19-vaccination/, Abdulsamad A. Aljeshi, et al. Case report and disucssion, read-only, 17 Feb. 2022
[22] https://doi.org/10.24869/psyd.2022.377, First Episode of Psychosis Following the COVID-19 Vaccination – A Case Series, Tonka Borodina, et al., 18 May 2022
[23] https://doi.org/10.3389/fpsyt.2024.1360338, New-onset psychosis following COVID-19 vaccination: a systematic review, Marija Lazarevna, et al., 11 Apr. 2024
[24] https://doi.org/10.3390/vaccines10101651, A Case Report: Multifocal Necrotizing Encephalitis and Myocarditis after BNT162b2 mRNA Vaccination against COVID-19, Michael Morz, 1 Oct. 2022
[25] https://doi.org/10.4103/indianjpsychiatry.indianjpsychiatry_607_22, Unmasking of schizophrenia following COVID-19 vaccination, Laxmi, Raj; Grover, Sandeep, Mar. 2023