The above image of lab equipment is courtesy of Google Images and Public Domain Pictures.
Today’s offering for Health Friday concerns what is called self-amplifying RNA (saRNA.) As the presentation includes discussion of saRNA COVID-19 “vaccines”, this post is dedicated to the memory of Yours Truly’s cousin Bill, who “died suddenly and unexpectedly” in September 2023.
Readers already know about the Important Wolf Moon Notifications, the importance of Civil Discussion, the Rules of our late, good Wheatie, and the caveats regarding Health Friday posts by Yours Truly. Links to these items can be found here. NOTE: Since this post is detailed and there are multiple areas to cover, Yours Truly has added Summaries of certain sections, and a General Summary at the end.
Before one begins: It is well known that the modRNA and the viral vector COVID-19 “vaccines” currently in use have been, and are, a disaster on multiple fronts. Not only do they induce literally thousands of negative medical and psychological effects in the bodies of those who took them and who take them now; these “vaccines” also cause death. The numbers of COVID-19 “vaccinated” persons presenting with COVID-19 “vaccine”-induced illnesses, injuries, disabilities, or “died suddenly and unexpectedly” are increasing by the month. Nobody knows exactly how long, or in what amount, the elements and mechanisms of the COVID-19 “vaccines” work in the “vaccinated” person’s body: what IS known, is that whatever “protection” is conferred by these “vaccines” is short-lived, while, at the same time, the “vaccine” mechanisms linger on in the body for an indeterminate period of time. Yours Truly has written extensively for this board regarding this situation. For further information, please see websites such as these: https://kirschsubstack.com/ (Steve Kirsch); https://petermcculloughmd.substack.com/ (Peter McCullough, MD); and, https://phinancetechnologies.com/HumanityProjects/Projects.htm#Nav_ExcessDeaths (Ed Dowd, statistician.)
At the same time, the development of new types of COVID-19 “vaccines”, as well as new types of “vaccine delivery” (intranasal, oral, and aerosol, for examples) goes on apace. One of the “newest” types of COVID-19 “vaccines” uses what is called self-amplifying RNA, or saRNA.
The concept of saRNA is the use of a small amount of RNA (or mRNA) in an injectable. Once introduced into the body of the patient, the saRNA theoretically goes to work, “re-creating itself.” (Think RNA or mRNA being turned into a “Xerox copier” inside the “vaccinated” person’s body.) The “goal” of saRNA is for the agent to “re-create itself” inside the “vaccinated” person’s body for a certain amount of time and in some amount. The patient’s body is “instructed” by the saRNA to “recognize” and produce antibodies against certain “enemies”, such as viruses. The theory is that a smaller amount of saRNA initially introduced into the body, followed by the “Xerox copier effect”, then followed by “instructing” the body to “recognize” and fight off certain “enemies”, will make saRNA a “more effective use” of mRNA in injectables.
Basically, saRNA COVID-19 “vaccines” turn the “vaccinated” person’s body into a “Xerox copier” (which, apparently, the “vaccinated” person’s body CANNOT stop, slow down, or mitigate) for the ingredients (and, by extension, the mechanisms) of the saRNA “vaccine.” Today’s post is a primer about saRNA COVID-19 “vaccines.”
The trail begins here, with these: www.science.org/content/blog-post/first-self-amplifying-mrna-vaccine, “The First Self-Amplifying mRNA Vaccine”, 25 January 2024, by Derek Lowe; https://jessicar.substack.com/p/why-we-cant-move-forward-with-self. “Why we can’t move forward with self-amplifying RNA technology”, by Jessica Rose, PhD, 7 September 2024; www.2ndsmartestguyintheworld.com/p/japans-plan-to-destroy-the-world, by Daniel Nagase, MD, 10 September 2024; and, www.freethink.com/health/sarna-vaccines, “World’s first “self-amplifying” vaccine approved in Japan”, by Kristin Houser, 16 December 2023. Please look at any or all of these to gain information about saRNA “vaccines.”
In addition, there is Dr. Robert Malone’s take: please see: https://x.com/newstart_2024/status/1840796021166600635. Below are screenshots of his remarks:


There are “pros and cons” regarding the use of saRNA in injectables. Below, from an article that discusses these, via www.promegaconnections.com/how-do-self-amplifying-vaccines-work/, by Jordan Nutting, 6 February 2024:


****** Summary: In other words, saRNA injectables **may**, at some point down the road, have **some** benefits. In the meantime — NONE of these types of injectables (GEMCOVAC, Kostaive) have been SUFFICIENTLY AND THOROUGHLY INVESTIGATED AND TESTED FOR USE ON HUMANS. But they are being approved ANYWAY, and are being injected into the bodies of unsuspecting persons who buy into the “Look, this shot has less mRNA than the ones you took before, and it’ll work better!” hype.
Here is a graphic depicting how saRNA works in the body of the person who is “vaccinated” with an saRNA injectable, via this “cheerleader” article: www.genscript.com/the-future-of-vaccination-unleashing-the-power-of-self-amplifying-rna-technology.html, by Dr. Zhen Sun, Editor, 9 May 2024.
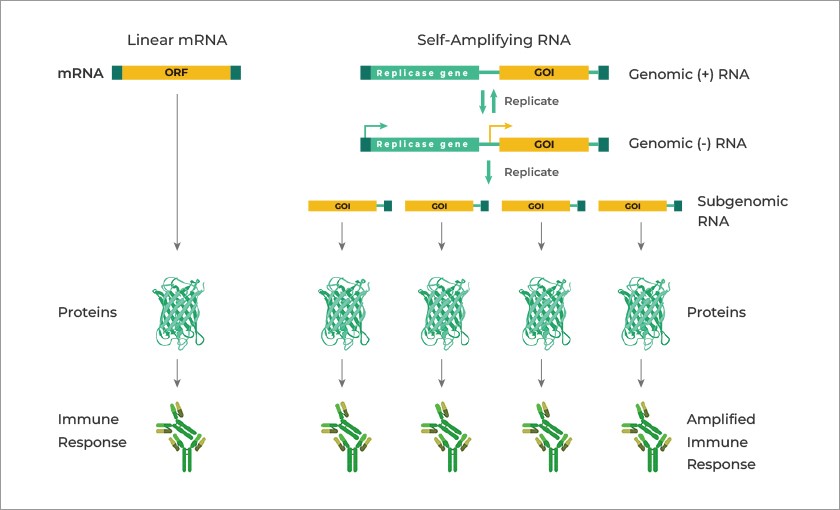
****** Look closely at the above graphic. It appears that the basic schema of saRNA is a “double-layer” of saRNA “replicons” that create a “subgenomic RNA.” This, in turn, creates the “Xerox copier” response which forces the body of the saRNA “vaccinated” person to endlessly produce immune system response — and for “at least” as long as 28 days after such “vaccination.”
Please see this paper regarding a discussion of saRNA “vaccine” design: https://doi.org/10.1016/j.tibtech.2023.05.007, “Rise of the RNA machines — self-amplification in RNA vaccine design”, Jerome D.G. Comes, et al., 14 June 2023. Below is the Abstract of this paper:

Another “cheerleader” article about saRNA “vaccines” is here: www.technologyreview.com/2024/02/1087536/the-next-generation-of-mrna-vaccines-is-on-its-way/, by Cassandra Willyard, February 2024. Below is a portion of the article:

Note the language about “,…at least in theory” advantage of saRNA “vaccines.” Also note the last sentence — saRNA can “persist for a month.” In fact, nobody really knows how long saRNA elements will “persist” in the body of the person who takes this type of “vaccine” — NO long-term clinical trial or study has been performed using this technology.
On 28 November 2023, the Japanese Government approved the use of the saRNA COVID-19 “vaccine”, ARCT-154 (also called LUNAR-COVID-19 and Kostaive) for “active immunization” (translation: “prevention”) against COVID-19 for persons age 18 and older. Kostaive was developed by CSL / Arcturus (remember this company? Yours Truly took the lid off it here: www.theqtree.com/2024/08/02/the-hhs-gave-the-go-ahead-to-use-an-h5n1-vaccine-but-the-ama-just-issued-new-cpt-codes-for-an-h5n8-vaccine/.) This “vaccine” was to have supplies ready for administration by physicians or hospitals by mid-December 2023.
CSL / Arcturus, the Japanese government, and media outlets were quick to herald this “first-ever saRNA COVID-19 “vaccine.” Except — there already was an saRNA COVID-19 “vaccine” approved and in use, since 2022, in India: GEMCOVAC. Perhaps what CSL / Arcturus, the Japanese government, and media outlets should have mentioned the fact that Kostaive is modRNA-based from the J.1. Omicron SARS-CoV-2 variant (along with other “familiar” manufacturing methods, see below in today’s post); whereas GEMCOVAC based on an “ancestral variant” (in other words, the Beta variant) of the original Wuhan Hu1 SARS-CoV-2 virus.
Before Yours Truly presents information on Kostaive, she will first discuss GEMOCOVAC. This is in order to present further background information on saRNA technology as applied in COVID-19 “vaccines.” Stay with me — this is all germane to the situation:
Looking at GEMCOVAC, one can get the beginning of a picture of how saRNA COVID-19 “vaccines” work. The “latest version” of this “vaccine” is called GEMCOVAC-OM. Below is a portion of the SmPC pdf for this product (https://gemcovac.com; scroll down the page to GEMCOVAC-OM SmPC (pdf) and click “Download”):
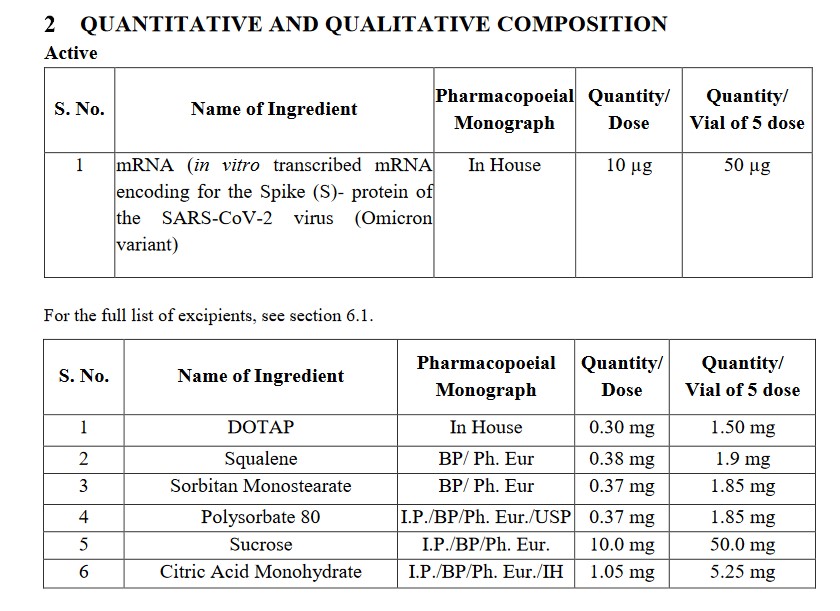
The BA.1. Omicron variant of SARS-Co-V-2 is the basis for this “vaccine.” It is not strictly modRNA (however, recall that ALL of the “descendant variants” of the original Wuhan Hu1 SARS-CoV-2 virus contain elements of that original lab-created virus).
Of the excipients (in other words, the adjuvants): per Wikipedia, DOTAP (1,2-Dioleoyl-3-trimethylammonium propane) is a chemical used in fabric softeners, but also is used as a lipid nanoparticle in vaccines. Squalene: below is Page 1 of the FisherScientific MSDS Safety Data Sheet for this chemical (www.fishersci.com/):
Continuing: for polysorbate 80, again from Fisher Scientific, Page 1 of the MSDS Safety Data Sheet:
And, here is the Mechanism of Action for GEMCOVAC-OM, from the Package Insert (see the link above):
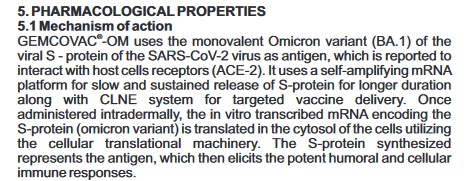
Note the language, “…which is reported to interact with host cells receptors (ACE-2.)” (Italics mine.) The developer and manufacturer (GENNOVA) of this “vaccine” can’t exactly quantify how the product works.
Yours Truly now turns to Kostaive, (ARCT-154) the saRNA COVID-19 “vaccine” that was approved by the Japanese government in November 2023.
The announcement regarding the Japanese government’s approval of Kostaive is here: www.meiji-seika-pharma.com/pressrelease/2023/detail/pdf/231128_01.pdf, of 28 November 2023. Below is a screenshot from the press release:
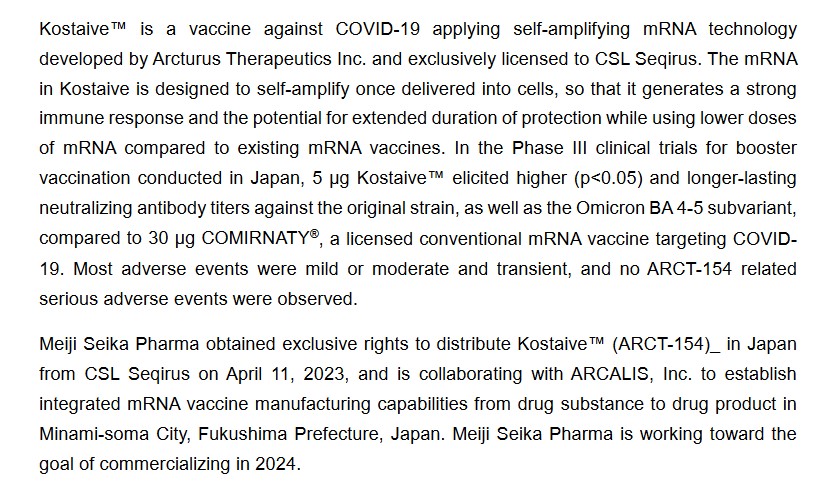
Note the language in the above regarding “…the potential for extended duration of protection while using lower doses of mRNA compared to existing mRNA vaccines.” (Italics mine) Again, nobody knows exactly how long this “extended duration” period is; nobody knows exactly how much “Xerox copying” of the altered mRNA in Kostaive occurs during this period in the “vaccinated” person’s body; and, nobody knows exactly what effects this “Xerox copying” of the altered mRNA in Kostaive will have in the “vaccinated” person’s body. In other words, anyone who takes Kostaive, or any saRNA “vaccine”, in Yours Truly’s opinion, is being used a “human lab rat” — just as people were used / are still being used, as “human lab rats” for the modRNA COVID-19 “vaccines.”
And, here is the Report of the Deliberation Results (which contains the information about Kostaive that led to its approval) from the Japanese Ministry of Health, Labour, and Welfare: www.pmda.go.jp/files/000269813.pdf. This report is an interesting read. There are numerous “blacked-out” areas, reminding one of the blacked-out areas in certain publicly-released Pfizer-BioNTech reports that the company gave to the FDA regarding that company’s modRNA COVID-19 “vaccine” BNT162b2. HOWEVER, section 2.1 Active Substance for Kostaive states that this saRNA “vaccine” contains elements from the original Wuhan Hu1 SARS-CoV-2 virus, plus elements from the Omicron variant strain. More information on this is found on Page 4 of the document. Below is a portion of this page:
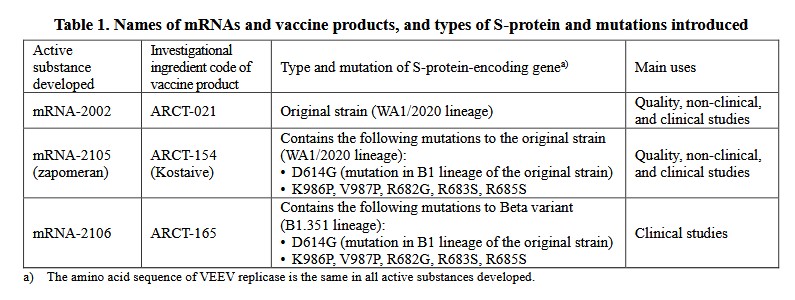
Looking at the above, starting with the D614G mutation, the Abstract from the Zhang, et al. paper on this (https://doi.org/10.1038/s41467-020-19808-4, “SARS-CoV-2 spike-protein D614G mutation increases virion spike density and infectivity”, Lizhou Zhang, et al., 26 November 2020. The paper was researched and published before any COVID-19 “vaccine” was authorized for use):
In other words, the D614G element causes the SARS-CoV-2 virus to be more infective.
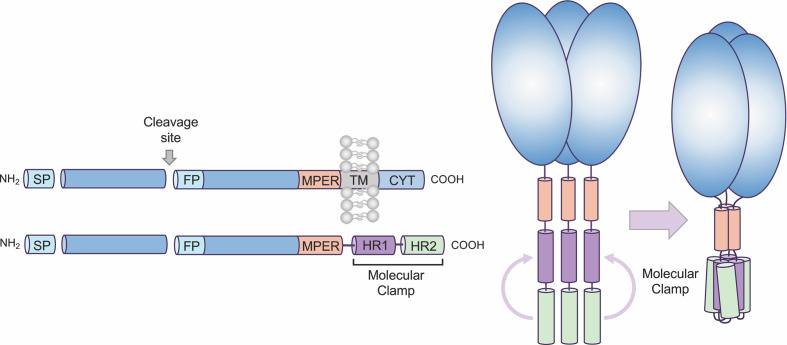
Looking at V987P, part of the section Full-Length S Glycoprotein Vaccines from this paper: https://doi.org/10.3389/fimmu.2021/701501, “SARs-CoV-2 Vaccines Based on the Spike Glycoprotein and Implications of New Viral Variants”, Daniel Martinez-Flores, et al., 11 July 2021):
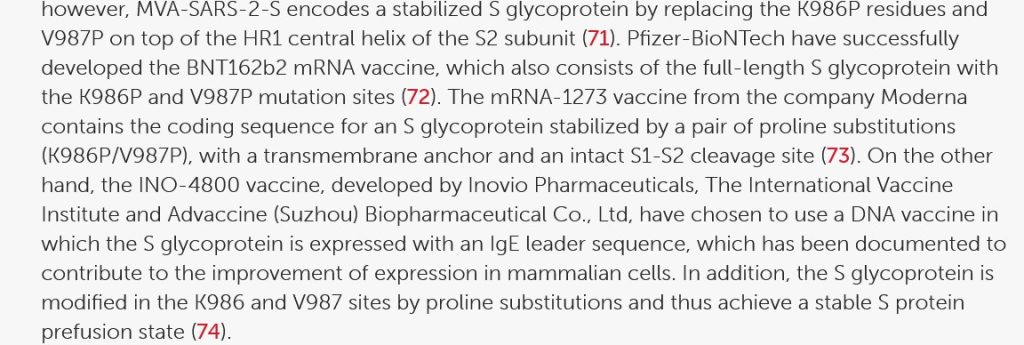
In other words, if V987P was “good enough” for Pfizer-BioNTech and for Moderna to use in their modRNA COVID-19 “vaccines”, it apparently was “good enough” to be used in Kostaive.
The K986P protein used in Kostaive: please see here: www.rcsb.org/structure/6zp1. This viral protein, along with V987P above, are BOTH on the Arg S1/S2 cleavage site on the SARS-CoV-2 virus genome. (FURIN CLEAVAGE SITE, anyone?)
The R682G, R683S, and R685S proteins used in Kostaive: please see here: https://doi.org/10.1038/s41467-022-32665-7, “Omicron SARS-CoV-2 mutations stabilize spike-up RBD conformation and lead to a non-RBM-binding monoclonal antibody escape”, Zhennan Zhao, et al., 24 August 2022. If appears that these proteins help to create a “one-RBD-up conformation.” Below is a portion of the Abstract of this paper:

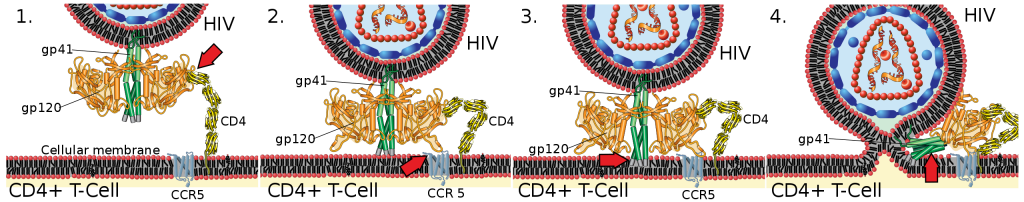
In other words, these proteins increase the immune system attack from the Omicron SARS-CoV-2 variants by making Omicron “stick better” to the ACE2 receptor cells in the human body.
****** Summary: It appears, then, that Kostaive contains one protein from the original Wuhan Hu1 SARS-CoV-2 virus genome (D614G); two proteins from the “ancestral line” of the SARS-CoV-2 virus (K986P and V987P); and three proteins of the Omicron SARS-CoV-2 variant (R682G, R683S, and R685S.) See below in the post for more information on these items.
One more ingredient of Kostaive, mRNA-2105: This ingredient is derived from the Venezuelan Equine Encephalitis virus (VEEV.) Below is section 4.R.1. of the Deliberations Results document discussing this:
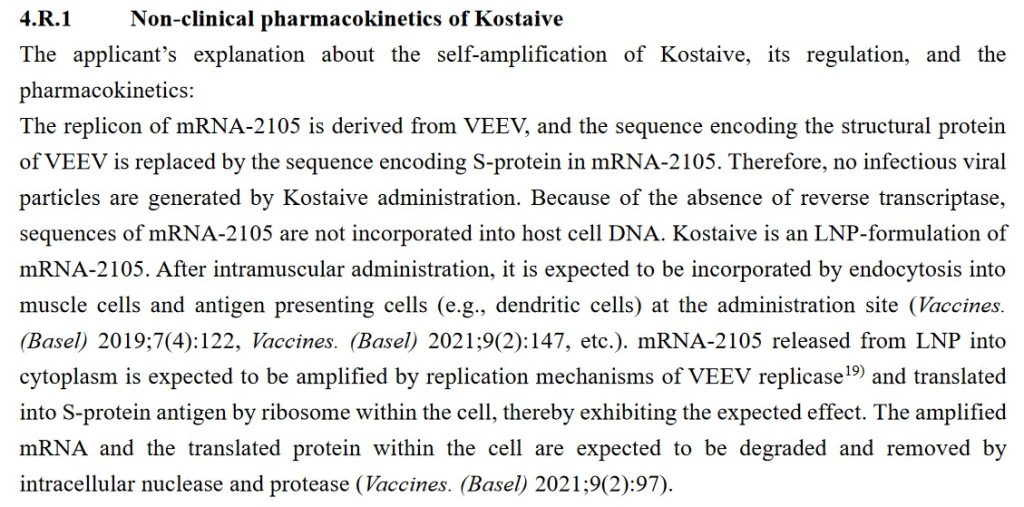
The question that immediately occurs regarding the above: How can Arcturus (the manufacturer of Kostaive) state that mRNA-2105 cannot be “incorporated into host cell DNA”? How is Arcturus absolutely sure that there is zero reverse transcription potential or ability in Kostaive? This is similar to the same statements from Pfizer-BioNTech about BNT162b2 “cannot change the DNA of the vaccinated individual” — which has been proven to be false (please see: here, Slide 14.)
****** Summary of the above: What Yours Truly is getting at here is that Kostaive appears to be the end-product of: ONE: a lab-created mixture of the dangerous D614G element from the original Wuhan Hu1 SARS-CoV-2 virus, plus, various elements of earlier SARS-CoV-2 mutations; TWO: NO long-term clinical trials, NO safety studies for Toxicity, use on pregnant women, etc.; THREE: the “Process 2”-type manufacturing method (“culturing” the lab-enhanced mRNA for the “vaccine” in a “bath” of E. coli); FOUR: using lipid nanoparticles, one of which (ATX-126) has never been used before in an injectable); FIVE: what appears to be a “pro-forma” Deliberation Results document on Kostaive that raises more questions than it answers; and, SIX: approval by the Japanese government for use on humans without a thorough investigation of the above. Related to point SIX: below is the list of Approval Conditions that were imposed along the approval of Kostaive for use in Japan, from the Deliberations Results document:

By the way, these Approval Conditions read very much like the ones that the FDA imposed on Pfizer-BioNTech along with that agency’s EUA for BNT162b2.
Regarding Kostaive itself, more particulars:
The ingredients of Kostaive are listed in section 2.2 Vaccine Product of the report. A screenshot of this section is below:
Kostaive contains at least three separate types of LNPs (lipid nanoparticles): ATX-126; DSPC; and, PEG2000-DMG. Below is a portion of the MSDS Safety Data Sheet for ATX-126:

Section 2.2.3 of the Deliberation Results document for Kostaive discusses the manufacturing process for Kostaive. It appears that Kostaive ALSO “switched” from a “Process A” manufacturing method over to a “Process B” manufacturing method. (Recall that the Pfizer-BioNTech BNT162b2 was “switched” from its “Process 1” manufacturing method over to a “Process 2” manufacturing method):
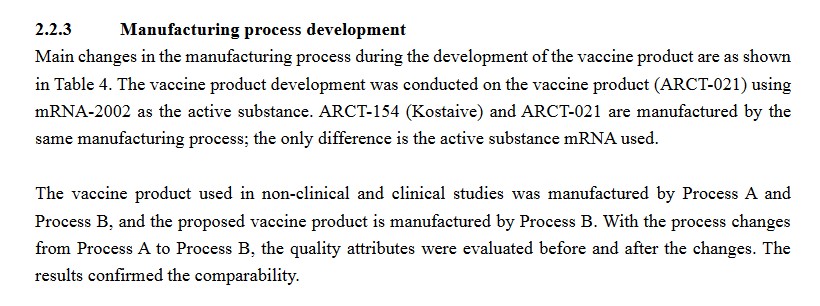
One more item from the Deliberation Results document for Kostaive, again on the ATX-126 lipid nanoparticle used in this “vaccine.” It confirms that this “novel excipient” (adjuvant) has not been used before in a “vaccine.”

Yours Truly will again emphasize that other sections of the Deliberation Results document on Kostaive make it clear that NO studies were performed for numerous items, such as Toxicity, potential for impairment of reproduction, and so on. By the way, buried in the document is a “passing reference mention” that the “Process B” manufacturing method for this “vaccine” uses E. coli as the “culturing medium” for the “enhanced” mRNA in the product. (The “Process 2” manufacturing method for BNT162b2 and its “descendant” COVID-19 “vaccines” also uses E. coli.) It also appears that the use of Kostaive on pregnant women in clinical trials was “inconclusive”; this “vaccine” should be taken by pregnant women only if the situation so warrants.
Also: Kostaive is to be taken as a “primary series” of two separate injections, 28 days apart; with a “booster” taken about three months later. The question that arises is: If it is true that the saRNA in Kostaive is active, including installing numberless “Xerox copiers” of itself in the “vaccinated” person’s body for AT LEAST 28 days AFTER the initial injection, WHY is there a need for ANOTHER injection around Day 28? And a “booster” after that?
****** BUT — AND THIS IS A HUGE BUT — TAKE ANOTHER LOOK AT SECTION 2.2.1 OF THE DELIBERATION RESULTS DOCUMENT FOR KOSTAIVE:

Look at the “active ingredient” item, “zapomeran.” What is zapomeran? It is a “drug” that contains the RNA of the Venezuelan Equine Encephalitis Virus (VEEV or VEE) in an saRNA form. It is ALSO manufactured by ARCTURUS (CSL.) It is unclear if it is being used in other types of COVID-19 “vaccines” other than Kostaive. Please see: https://synapse.patsnap.com/drug/510bcf7ef75649278b284a94663c69f6. Scroll down the page to R&D Status to see that zapomeran has already been approved in the EU; Norway; Iceland; and Liechtenstein; but in what final form it is used, is also unclear. NOTE 1: It has been impossible to find a complete list of ingredients for zapomeran. One would not be surprised to learn that the names Kostaive and zapomeran may, in some respects, be “interchangeable.” NOTE 2: It is not easy to find information on zapomeran. Yours Truly has encountered “504 Bad Gateway” error messages when going back to recheck a couple of the links to zapomeran in this post.
Looking further into VEEV (or VEE), there is this article: https://ceh.vetmed.ucdavis.edu/health-topics/venezuelan-equine-encephalitis-vee, by Amy Young, 28 August 2020. This virus can affect horses, donkeys, or zebras. VEEV is transmitted to these animals by infected mosquitos that bite them. It can cause severe disease or death by infecting the brain and the central nervous system. Humans who contract VEEV can also become severely ill and can also die from it. Another paper, discussing the RNA of VEEV (VEE),by Sarah E. Hickson and Jennifer L. Hyde, is here.
As an aside: zapomeran (under the name “ARCT-154-06”) was granted a “deferral” for use on children from birth to 18 years of age in the European Union for the “prevention” of COVID-19 infection in JUNE 2023: www.ema.europa.eu/en/documents/pip-decision/p-0204-2023-ema-decision-5-june-2023-agreement-paediatric-investigation-plan-granting-deferral-zapomeran-emea-003349-pip01-22_en.pdf. This means that the use of zapomeran (in whatever form) is restricted to persons age 18 and older.
AND HERE IS THE JAPANESE NIHS LISTING FOR ZAPOMERAN: https://jpdb.nihs.go.jp/jan/DetailList_en?submit-all_alpSearch&keyword=Zapomeran. Below is a screenshot of the listing (scroll down past the genome sequences listings):

****** VERY IMPORTANT: The SARS-CoV-2 spike proteins in zapomeran are the same ones that are in Kostaive. These were discussed above in the post. Turning to the nsP proteins (non-structural proteins) in zapomeran (hyperlinks to papers are embedded), as these elements are also apparently contained in Kostaive:
nsP1: this non-structural protein suppresses the immune system; Katharina Schubert, et al.
nsP2: this non-structural protein is a “delivery vehicle” for SARS-CoV-2 proteins; Ninge Zheng, et al.
nsP3: this non-structural protein is a “vital component in the replication of SARS-CoV-2”; Sofia Lemark, et al.
nsP4: this non-structural protein is the largest one. It is a Rotavirus enterotoxin that causes diarrhea, can cause severe diarrhea, and particularly affects young children; Judith M. Hall, et al.
****** SUMMARY: IT APPEARS, THEN, THAT KOSTAIVE CONTAINS ZAPOMERAN, WHICH INCLUDES THE VENEZUELAN EQUINE ENCEPHALITIS RNA; PLUS, SIX LAB-ENHANCED/LAB-ISOLATED GENOME CODES OF SARS-CoV-2; AND, FOUR NON-STRUCTURAL PROTEINS, ONE OF WHICH IS A ROTAVIRUS TOXIN. KOSTAIVE ALSO CONTAINS DANGEROUS LIPID NANOPARTICLES (SUCH AS ATX-126.) THESE ARE ALL PRESENT IN THIS saRNA COVID-19 “VACCINE” PRODUCT. RECALL THAT LIPID NANOPARTICLES WILL HELP TO SPREAD A “VACCINE” THROUGHOUT THE ‘VACCINATED” PERSON’S BODY, INCLUDING CROSSING THE BLOOD-BRAIN BARRIER.
Why on Earth is a COVID-19 “vaccine” that contains the RNA of an equine brain inflammation virus (a virus that comes from infected mosquitos that bite equine animals) being used on humans? A “vaccine” that is engineered to create an unknown number of “Xerox copiers” of the “vaccine” elements into the body of the “vaccinated” person? A “vaccine” that contains six apparently lab-enhanced/lab-isolated genome codes from the SARS-CoV-2 virus or its variants? What does an equine brain inflammation virus have in common with SARS-CoV-2? What if a person who has already taken, say, five or six injections of a modRNA COVID-19 “vaccine” decides to take Kostaive? Could an saRNA COVID-19 “vaccine” somehow “interact” with the modRNA COVID-19 “vaccine” elements already in that person’s body?
****** GENERAL SUMMARY: saRNA “vaccines” for COVID-19 are already being used (GEMCOVAC in India); and are being approved for use (Kostaive in Japan.) The technology for saRNA is not fully developed and not fully tested; the COVID-19 “vaccines” that use saRNA contain “lab-enhanced”/”lab-isolated” genome codes of the SARS-CoV-2 virus or its variants; saRNA COVID-19 “vaccines” contain lipid nanoparticles — and, in the case of Kostaive, an LNP (ATX-126) that has never been used before in an injectable; that no studies have been performed on saRNA COVID-19 “vaccines” regarding Toxicity, the effects on reproductive potential, and so on; and, that Kostaive contains the RNA of the Venezuelan Equine Encephalitis Virus. And yet, these products are being hailed as “the vaccines of the future.” And, the people taking them are again being used as “human lab rats.”
Peace, Good Energy, Respect: PAVACA