A background research post by Gail Combs
PREFACE
by Wolf Moon
It is useful for me to explain Gail’s post – to help you understand the importance of it.
People in government regulation of science and medicine do not make decisions in a vacuum – but they may make their decisions in an artificial atmosphere which is created, composed, and altered by those with the extreme power and ability to CONTROL INFORMATION.
LancetGate was very demonstrative of this ability to control science and medicine.
After watching a variety of FDA decisions under the Trump and Biden administrations, it has become very clear to me that FDA resides in a political and economic crosswinds, highly influenced by many parties with strong expectations and abilities to influence. And yet, the shocking (but welcome) advisory recommendation AGAINST COVID vaccine boosters – then overridden by the political operative Rochelle Alinsky Walensky in CDC – shows how a coordinated injection of honest medicine and common sense into FDA decision-making (THANK YOU, Steve Kirsch!) can sometimes make its case heard – even if only momentarily.
Sadly, it seems to me that Pfizer is back in the driver’s seat again. We thus have to ask WHY.
What Gail has done is to look at one endpoint of the argument (frontline doctors and publishing scientists who have run into the problems), which leads to the other, where we begin to find the reasons why FDA generally decides things in favor of big industry and big government, and not to the benefit of individual patients and doctors.
By looking at the doctors and scientists who supported logical and ethical TREATMENT of COVID-19, and who were wrongly and unethically BLOCKED and DENIED PERMISSION at every point – we can see that undue industry and political influence in NIH, CDC, and FDA are most likely responsible for decisions that make absolutely no sense to truly independent scientists and doctors. The video Gail includes is rather astounding in terms of showing us how much went wrong. What we are now seeing reminds me of science and medicine in the Soviet Union. Absolutely incredible.
What Gail has done here is to “follow the influence” – to show that FDA decision-making has NOT been in a vacuum, precisely because the members of the FDA advisory committee are not truly independent, but are in fact actors for the very same powerful forces that benefit from FDA decisions which are now inscrutable at the doctor-patient frontline.
Perhaps even more astoundingly, the very SYSTEM of NIH, FDA, and CDC seems to be designed, at this point, to leave NOBODY ACCOUNTABLE. Advisory groups and even department responsibilities are created, rearranged, and dismissed, so that NOBODY takes responsibility for mandates that defy logic and even violate the common medical sense of the past.
If something goes wrong in this chaotic system of responsibilities in the wrong places, you either blame everybody or nobody at all. This is why, in my opinion, the entire federal governent had to get rid of Trump.
DIG ALONG WITH GAIL, as she finds the CLUES – first in the testimony of Ron Johnson’s witnesses – then in the backgrounds of members of an important FDA advisory panel.
With that, here’s Gail.
The Covid Second Opinion Forum
It would be nice to let Senator Johnson know we saw this:
CONTACT: https://www.ronjohnson.senate.gov/contact
Offices – Mail address and Phone: https://www.ronjohnson.senate.gov/office_locations
MANY THANKS TO GA/FL and Wolf M00n for alerting us to the Covid 2nd opinion Forum
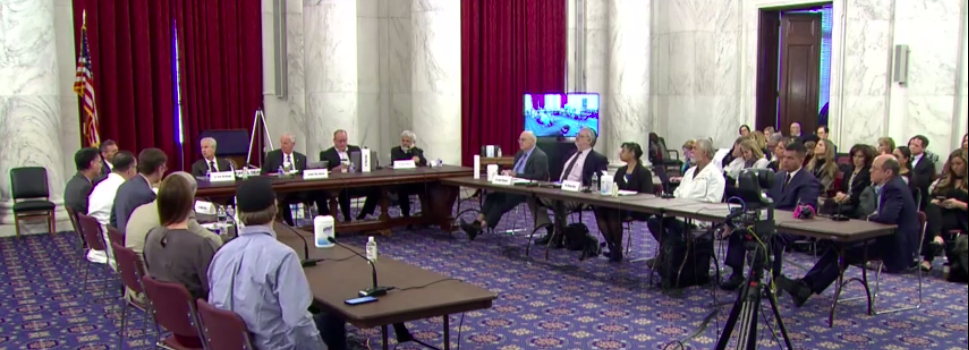
Here’s a must watch – A SECOND OPINION – SENATOR RON JOHNSON FORUM.
Begins at 40:19 – https://rumble.com/vt62y6-covid-19-a-second-opinion.html
“Discussion begins around 40 minute mark. Sen. Ron Johnson moderates a panel discussion, COVID-19: A Second Opinion. A group of world renowned doctors and medical experts provide a different perspective on the global pandemic response, the current state of knowledge of early and hospital treatment, vaccine efficacy and safety, what went right, what went wrong, what should be done now, and what needs to be addressed long term.”
More at https://www.ronjohnson.senate.gov
GA/FL
It is five hours long and I went through the whole video. I recommend listening to it as you work on other things since there is not much to watch. It is much better than the speeches at the March Against Mandates.
My, comments posted 1/25/2020:
1:41:50 – 1:50:00 Dr Paul Marik on Remdesivir:
4 million hospitalized 850,000 million died. He says cheap approved drugs could have save 500,000 lives (That is the 1/2 million again that I figured out by a different method.)
He eviscerated NIH/FAUCI.
Fauci Told POTUS Remdesivir was good 10 days in. Researchers Changed the study END POINT to HIDE DEATHS. The OTHER study SHOWING the deaths/toxicity of Remdesivir in Ebola trial was released at 11:00 am JUST before the Afternoon presentation of the corrupted Remdesivir-Covid study that was presented by Fauci to POTUS. (More on this below) He also mentioned U.S. Centers for Medicare & Medicaid Services gives bonuses to hospital to treat MEDICARE (the old and ‘useless’) patients with this toxic drug.
Steve Kirsch made it clear he thought it was corruption and worse several times.
Incriminating evidence – Steve Kirsch’s newsletter
New VAERS analysis reveals hundreds of serious adverse Events that the CDC and EDA Never Told Us About
3:12:00 MyFreeDoctor (dot)com:
This group treated 150,000 and only lost four.
3:35:00 Dr Peter McCollough talks about vaccines:
Originally there were three different advisory boards during the trials but when it came to the EUA those boards were gotten rid of AND THAT IS WHY CLOT SHOT WAS NOT PULLED IN JANUARY 2021!
Steven Kirsch says right after that there is HARD evidence at least 4 in the CDC/FDA were getting royalties…
4:02:00
The risk increases with each shot. mRna was ENGINEERED to TAMP DOWN RESPONSE to evade ADE BUT it looks like it ALSO tamps down response to viruses, bacteria, and cancer.
New Study Dr Voss out of the Netherlands.
There are too many Dr Voss in the netherlands for me to hunt down the correct one.
https://pubmed.ncbi.nlm.nih.gov/?term=voss%20netherlands&sort=date
(Could be KL Koss since she has papers about the heart and colon and cancer. papers: https://pubmed.ncbi.nlm.nih.gov/?term=Koss+KL&cauthor_id=8943944
4:43:00 Attorney Tom Renz:
He has Dept of Defense Whistleblowers with the data from the D-Med data base. They have taken data ‘snapshots’ over time and it shows THE DATA BASE WAS TAMPERED WITH TO HIDE THE INJURIES TO OUR SOLDIERS!
Senator Johnson Ordered PRESERVE YOUR RECORDS….
January 25, 2022 14:52
Apparently Daniel Horowitz chased down Attorney after the Ron Johonson Senate Hearing for some additional clarifications.
https://thelibertydaily.com/bombshell-cover-up-cancer-diagnoses-in-the-military-rose-over-three-fold-since-jabs-were-introduced/
para59r
5:05:00
Myocarditis and heart Hits 18 to 24 yr old males the worst. up to 50 years 21,000 cases so far.
A lot more good info.
Dr. Christina Parks made comments through out the presentation.
January 25, 2022 20:09
Yes – Dr. Christina Parks has made some excellent points about how differently people with African genetic background react to CV19 AND THE VACCINES.
Ethnicity does matter in medicine – my sister had concurrent dengue fever and malaria and her response was severe and peculiar to a certain ethnicity so….we learned may have some middle eastern/african blood somewhere previously unknown to us.
GA/FL
The Timeline of FDA Decisions
Heading down the Rabbit Hole on Vaccines that Dr Peter McCollough opened.
Emergency Use Authorization — FDA
As background, this gives the laws, who has the authority and the timeline.
TIME LINE:
October 13, 1976 – New York Times:
Swine Flu Program Is Halted in 9 States As 3 Die After Shots
“After the deaths, swine flu vaccinations were halted throughout Allegheny County, which includes Pittsburgh, and the Federal Center for Disease Control sent doctors to investigate….“
September 1, 2020 CNN
Past vaccine disasters show why rushing a Covid-19 vaccine now would be ‘colossally stupid’
This is actually a very good article on BAD vaccines and what it can do to the public’s trust.
And then we come to the FDA, the meetings and WHO is on the board.
October 22, 2020
Discussing, in general, the development, authorization and/or licensure of vaccines to prevent COVID-19
On October 22, 2020, the Center for Biologics Evaluation and Research’s (CBER), Vaccines and Related Biological Products Advisory Committee (VRBPAC) will meet in open session, to discuss, in general, the development, authorization and/or licensure of vaccines to prevent COVID-19.
FDA
Those are probably the three boards Dr Peter McCollough talks about. The third was the FDA Vaccines and Related Biological Products Advisory Committee that hold these meetings. Note they are meeting just before the election and it contains ALL three boards.
….
These meetings are AFTER the STOLEN ELECTION but again all three advisory boards are present.
December 10, 2020
Discussing First Emergency Use Authorization Request for a COVID-19 Vaccine
On December 10, 2020, the Center for Biologics Evaluation and Research’s (CBER), Vaccines and Related Biological Products Advisory Committee (VRBPAC) will meet in open session to discuss Emergency Use Authorization (EUA) of the Pfizer-BioNTech COVID-19 Vaccine for the prevention of COVID-19 in individuals 16 years of age and older.
FDA
December 17,2020
Discussing Second Emergency Use Authorization Request for a COVID-19 Vaccine
Agenda
The meeting presentations will be heard, viewed, captioned, and recorded through an online teleconferencing platform. On December 17, 2020, the Center for Biologics Evaluation and Research’s (CBER), Vaccines and Related Biological Products Advisory Committee (VRBPAC) will meet in open session to discuss Emergency Use Authorization (EUA) of the Moderna, Inc., COVID-19 Vaccine for the prevention of COVID-19 in individuals 18 years and older.
FDA
December 15, 2020 updated December 18
The ACIP met last week to review the Pfizer-BioNTech vaccine and recommended moving forward with its distribution to anyone over age 16. The FDA issued an EUA on Saturday following the meeting and notified the CDC and Operation Warp Speed to coordinate distribution plans. Initial doses were shipped over the weekend. The first round of deliveries will be completed in all 50 states this week…
Pfizer’s initial distribution of vaccines will be given to 21 million health care workers and 3 million mostly elderly people living in long-term care facilities.
As vaccines are deployed, data on potential or delayed side effects will be collected to answer questions that would have been addressed in long-term trials with millions of participants under nonemergency circumstances….
Nat’l Conf. of State Legislators
December 14, 2020, 8:33 PM – Black nurse in New York, Sandra Lindsay, gets the first vaccine.
A month later we get the first Adverse Reaction Reports.
January 18, 2021 – Coronavirus: California calls for pause, investigation after Allergic Reactions to Moderna Vaccine
Biden is installed in the White House and the FDA/CDC does no real investigation. Instead NOTE THE CHANGE IN MEETING MINUTES.
February 26, 2021
Discussing Third Emergency Use Authorization Request for a COVID-19 Vaccine
Agenda
The meeting presentations will be heard, viewed, captioned, and recorded through an online teleconferencing platform. The committee will meet in open session to discuss EUA of the Janssen Biotech Inc. COVID-19 Vaccine for active immunization to prevent COVID-19 caused by SARS-CoV-2 in individuals 18 years and older. <== NO ADDITIONAL ADVISORY BOARDS!
Meeting Materials
FDA intends to make background material available to the public no later than 2 business days before the meeting. <== THIS IS THE INFORMATION people are having to SUE FOR!
FDA
Note there are NO MEETINGS TO DISCUSS DEATHS OR ADVERSE REACTIONS! This is the NEXT MEETING:
Discussing Pediatric Use of COVID-19 Vaccines
The Committee will meet in open session to discuss, in general, data needed to support authorization and/or licensure of COVID-19 vaccines for use in pediatric populations.
Meeting Materials
FDA intends to make background material available to the public no later than 2 business days before the meeting. If FDA is unable to post the background material on its website prior to the meeting….
FDA
Now we come to the meat, exactly who is at those meetings?
The VRBPAC Advisory Committee
The Committee reviews and evaluates data concerning the safety, effectiveness, and appropriate use of vaccines and related biological products which are intended for use in the prevention, treatment, or diagnosis of human diseases, and, as required, any other products for which the Food and Drug Administration has regulatory responsibility. The Committee also considers the quality and relevance of FDA’s research program which provides scientific support for the regulation of these products and makes appropriate recommendations to the Commissioner of Food and Drugs.
The Committee shall consist of a core of 15 voting members including the Chair. Members and the Chair are selected by the Commissioner or designee from among authorities knowledgeable in the fields of immunology, molecular biology, rDNA, virology; bacteriology, epidemiology or biostatistics, vaccine policy, vaccine safety science, federal immunization activities, vaccine development including translational and clinical evaluation programs, allergy, preventive medicine, infectious diseases, pediatrics, microbiology, and biochemistry.
FDA
Applying for Membership on FDA Advisory Committees
As part of the Food and Drug Administration’s (FDA’s) ongoing efforts to recruit qualified experts with minimal conflicts of interest who are interested in serving on FDA advisory committees, FDA is requesting nominations for members to serve on its advisory committees….
Conflicts of Interest:
Potential candidates are asked to provide detailed information concerning such matters as financial holdings, employment, and research grants and/or contracts in order to permit evaluation of possible sources of conflict of interest.
FDA
Oooh Boy, they do not sound good. I am digging up and placing here a lot of info on each individual. However I have screened out much much more. What struck me, is out of the sixteen only three do not have major expertise in pediatrics. ALL have ties to NIH/Fauxi or the FDA or the CDC. Some have ties to drug companies and more than one has ties to China. Most are women and three are foreign educated and probably not American born. Out of over 300 million people, you would think they could find Americans.
First a cameo of each of the players, and then if you wish you can look at some of the other information I dug out. If you click on the name it takes you to the information they provided to the FDA, often pages and pages citing the papers they wrote and who they worked for. This is the information I used with some added from other sources.
CAMEOS
DIRECTOR
Prabhakara Atreya, Female connected to Fauci She has no FDA bio.
Ph.D. biochemistry Memorial University of Newfoundland, in Canada 1987
Wayne State University, School of Medicine, Detroit, MI 1989 – 1990 (messing with fiber cell membranes of frog, chick, bovine, rabbit and human lenses)
Plant Pathology, University of Kentucky, Lexington KY – Papers 1990 &1995
FDA since 2010 per BIO but actually a paper shows she was working @ FDA in 1999.
NIH paper shows she was at NIH in 1998
Plant Pathology, University of Kentucky, Lexington KY – Papers 1990 &1995 (Only 13 papers found)
…..
Chair
Hana El Sahly, M.D.
Baylor College of Medicine (Woman)
Wrote paper on Remdesivir used by Fauci to trick President Trump. The one referred to by DR. McCullough
…..
Paula Annunziato, M.D. ***
Vice President and Therapeutic Area HeadVaccines Clinical ResearchMerck
Seems to specialize in Pediatric Vaccines.
Archana Chatterjee, M.D., Ph.D.
Dean Chicago Medical School (Woman)
specialises Pediatrics Vaccines
Pune University, Maharashtra, India 1979-1983
Army Medical Corps, Military Hospital, Gaya, India, 1985 -1988
She is nationally recognized for her work in vaccine development for human papilloma viruses – think Gardasil. I wonder how well acquainted she is with Gregg Sylvester, below & Bill Gates? – Controversial vaccine studies: Gates and Infertility
…….
CAPT Amanda Cohn, M.D.
Expertise: Pediatrics, Vaccines
Chief Medical Officer – National Center for Immunizations and Respiratory Diseases – CDC
The mission of the National Center for Immunization and Respiratory Diseases (NCIRD) is the prevention of disease, disability, and death through immunization
59 Scientific papers: many authored with Nancy E Messonnier
Time to check the Atlantia graveyards… And I am NOT kidding.
…..
Hayley Gans, M.D. (woman)
Expertise: Pediatrics, Infectious Diseases
Department of Pediatrics
Stanford University Medical Center
Author with a bunch of Chinese with FUNDING from China, using the bogus PCR test to say people who have recovered can catch Covid again and re-infect others. This is WHY natural immunity was never on the table and vaccines were.
….
Holly Janes, Ph.D.
Expertise: Biostatistics
Professor – Fred Hutchinson Cancer Research Center
Vaccine and Infectious Disease Division
Division of Public Health Sciences – Seattle, WA
Holly is a biostatistician working on the design and analysis of vaccine studies, with a particular expertise in HIV prevention and vaccine science. Worked for NIH and Bill & Melinda Gates Foundation.
…..
Michael Kurilla, M.D., Ph.D.
Expertise: Infectious Diseases, Pathology
Director, Division of Clinical Innovation National Center for Advancing Translation Sciences
National Institutes of Health (NIH <– He works for Fauci)
Bethesda, MD 20852
….
Myron Levine, M.D., D.T.P.H., F.A.A.P
Expertise: Infectious Diseases
Associate Dean for Global Health – Vaccinology and Infectious Diseases
Center for Vaccine Development – University of Maryland School of Medicine Baltimore, MD
Faculty at CVD have served in critical leadership roles in U.S. and international research and policy efforts. For example, Neuzil co-chaired the COVID-19 Prevention Trials Network, a research network established by the National Institute of Allergy and Infectious Diseases [ Dr. Fauci was appointed director of NIAID in 1984.] in response to the pandemic. Vaccine research at CVD continues, with an emphasis on reaching the populations most impacted by COVID-19 and testing pediatric vaccines.
University of Maryland
….
H. Cody Meissner, M.D. (Male)
Expertise: Infectious Diseases
Professor of Pediatrics – Tufts University School of Medicine
Director, Pediatric Infectious Disease
H. Cody Meissner, MD, is a leading national expert on childhood vaccinations who consults with the Centers for Disease Control and Prevention on periodic updates to the recommended immunization schedule for newborns through 18-year-olds. At Tufts Children’s Hospital at Tufts Medical Center he heads the Division of Pediatric Infectious Diseases…
H. Cody Meissner, MD, Vice Chair (’19)
H. Cody Meissner, MD | Tufts Medical Center
…..
Paul Offit, M.D.
Expertise: Infectious Diseases
Professor of Pediatrics, Division of Infectious Diseases, The Children’s Hospital of Philadelphia
Paul A. Offit, MD is the Director of the Vaccine Education Center at the Children’s Hospital of Philadelphia as well as the Maurice R. Hilleman Professor of Vaccinology and a Professor of Pediatrics at the Perelman School of Medicine at the University of Pennsylvania. He is a recipient of many awards including the J. Edmund Bradley Prize for Excellence in Pediatrics from the University of Maryland Medical School, the Young Investigator Award in Vaccine Development from the Infectious Disease Society of America, and a Research Career Development Award from the National Institutes of Health. Dr. Offit has published more than 160 papers in medical and scientific journals in the areas of rotavirus-specific immune responses and vaccine safety….
FDA
In 2017, Dr. Offit was a weekly columnist for The Daily Beast.
A look at his recent peer-reviewed papers shows he is targeting vaccine hesitant parents.
https://pubmed.ncbi.nlm.nih.gov/?term=Offit+PA&cauthor_id=24011750&size=20
This says it all:
Plotkin SA, Offit PA, Reiss D.: Important New Resource for Clinicians Giving Expert Witness Testimony on Vaccines. Pediatr Infect Dis J. 37(12), Dec. 2018.
To the Editors:
Vaccination is under attack by individuals who occasionally use the legal system to oppose mandatory vaccination laws and in some cases to obtain exemptions for particular children whose parents are opposed to vaccination. During the legal proceedings, as we have witnessed, experts testifying in favor of vaccination may be challenged with references from journals of doubtful quality that oppose vaccination.
To provide important references that discuss and disprove claims made against vaccines, the Vaccine Education Center at the Children’s Hospital of Philadelphia has created a library of references addressing certain safety issues that may be useful as an aid and refresher to clinicians giving expert testimony on the safety of vaccines and to lawyers defending vaccination of children.
The Children’s Hospital of Philadelphia legal library may be entered through the web address vaccine.chop.edu/safety-references.
We would be grateful if you could inform your colleagues about the availability of this resource, which should be of great value for experts testifying for vaccination and for clinicians who need to convince parents about vaccine safety. https://journals.lww.com/pidj/Fulltext/2018/12000/Important_New_Resource_for_Clinicians_Giving.42.aspx
The Pediatric Infectious Disease Journal
………..
Steven Pergam, M.D.
Expertise: Infectious Diseases
Medical Director
Infection Prevention
Seattle Cancer Care Alliance — Seattle, WA
He seems to specialize in cancer and immuno-compromised and seems to be the best of a bad bunch, until you see he is tied at the hip to NIH & CDC from 2009 to present as well as to various drug companies.
….
Jay Portnoy, M.D.
Expertise: Consumer Representative (This is the guy representing the public)
Professor of Pediatrics
Medical Director of Telemedicine Section of Allergy, Asthma and Immunology
Children’s Mercy Hospital Kansas City, MO
150 papers mainly on allergies. American College of Allergy, Asthma & Immunology – “…a professional medical association of more than 6,000 allergist-immunologists and allied health professionals…” AND if you search long enough…. You find the American College of Allergy, Asthma & Immunology wrote an Article urging allergists to support more funding for NIH (Fauci)
He is also on a Task Force Paper recommending those with severe egg allergies to go ahead and get the Flu vaccine, just do it at the allergist because “… personnel to recognize and equipment to treat anaphylaxis need to be immediately available…”
….
Andrea Shane, M.D., M.P.H., M.Sc.
Expertise: Pediatric & Infectious Diseases
Professor of Pediatrics, Director Division of Pediatric Infectious Diseases – Emory University School of Medicine – Atlanta, GA
International exchange fellowship, Children’s Hospital at Montefiore and Beijing Children’s Hospital, Beijing, China October-November, 1999
Lieutenant Commander, United States Public Health Service, 2001-2003;
Inactive Reserve Corps (IRC) 2003-until IRC dissolved in 2010.
Centers for Disease Control and Prevention, Advisory Committee on Immunization Practices (ACIP) respiratory syncytial virus (RSV) immunoprophylaxis working group, appointed member, 2009-until committee dissolved by CDC in 2011.
01 August 2007- 01 August 2016 Co- investigator, NIH/NIAID/DMID Vaccine and Treatment Evaluation Unit (VTEU)
influenza vaccine to breastfeeding women trial, DMID#09-007;
…..
Paul Spearman, M.D.
Expertise: Pediatric & Infectious Diseases
Director, Division of Infectious Diseases
Albert B. Sabin Chair in Pediatric Infectious Diseases
Cincinnati Children’s Hospital Medical Center
Professor, Department of Pediatrics
University of Cincinnati School of Medicine Cincinnati, OH
This guy is a really big heavy weight.
There is cross-over with the lady above, Andrea Shane. Any bets he pulled her in to be his ‘female puppet’ – a good little government soldier?
03/2009-09/2016: Vice Chair for Research Department of Pediatrics Emory University School of Medicine
03/2009-09/2016: Chief Research Officer Children’s Healthcare of Atlanta Atlanta, GA
[Andrea Shane is Attending Pediatrician Children’s Healthcare of Atlanta Emory Healthcare Grady Health 2006 – present ]
This guy has a full page of COMMITTEE MEMBERSHIPS, National and International, and a whole section for NIH Councils and Study Sections AND… NIH/NIAID HIV Vaccine Trials Network – Protocol Chair, HVTN 088 Protocol 2010-present
Not to mention his connections to the drug companies and China.
………
Geeta K. Swamy, M.D.
Expertise: Infectious Diseases
Senior Associate Dean Vice Chair for Research & Faculty Development
Associate Professor, ObGyn, Department of Obstetrics & Gynecology, Division of Maternal-Fetal Medicine
Duke University, Durham, NC
2004 – 2006 Duke University Associate Faculty Department of Obstetrics & Gynecology Division of Maternal-Fetal Medicine & Division of Clinical & Epidemiological Research
2009 – present Duke University Vaccine Trials Unit Investigator Duke Translational Research Institute Durham, NC
Grants from, NIH-NIAID, GlaxoSmithKline, CDC-NCIRD, ACOG/Merck & Company,
2015
Consultative Workshop: Immunology Research Gaps Related to Maternal Immunization – Bill & Melinda Gates Foundation
WHO Brighton Collaboration Global Alignment of Immunization Safety Assessment in Pregnancy – Chair, Fetal Distress Working Group
…
Gregg Sylvester, M.D., M.P.H. +
Expertise: Alternate Industry Representative
Vice President – Medical Affairs, Seqirus Inc., Summit, NJ
• Launched Pfizer’s Pediatric and Adult Pneumococcal conjugate vaccine,
Spearheaded science-based rationale to preserve Pfizer’s Prevnar 13 infant schedule in US recommendations
• Launched Merck’s HPV4 vaccine in over 100 countries
• Partnered with community organizations in Delaware to reduce infant mortality, teen pregnancy rates and HIV rates
Created the medical affairs strategy for Merck’s HPV4 vaccine, Gardasil
….
Vaccine Approval For Children
Now, if you have time & stomach, a deeper dive into the people who unleashed the Clot Shot on babies.
DIRECTOR
Prabhakara Atreya, Ph.D.
Division of Scientific Advisors & Consultants
Center for Biologics Evaluation & Research
Food and Drug Administration – Silver Spring, MD
Dr. Prabhakara Atreya, an Indian American scientist is a 10 year veteran at the US Food and Drug Administration which she joined in 2010. Prior to this appointment, Atreya worked at the National Institutes of Health, leading the Office of Scientific Review. She has a PhD in biochemistry, biophysics and molecular biology from the Memorial University of Newfoundland, in Canada. She was one of the team of US regulators and independent experts of Vaccines and Related Biological Products Advisory Committee (VRBPAC). At the time of emergency use authorization for Pfizer’s Covid-19 vaccine, she was the Acting Designated Federal Officer of VRBPAC.
LINKED-IN
Thesis:
Atreya, Prabhakara Lakshmi (1987) Conformational aspects of proline hydroxylation in collagen biosynthesis : studies with synthetic peptides. Doctoral (PhD) thesis, Memorial University of Newfoundland.
Probable Papers (13):
Affiliation: Department of Plant Pathology, University of Kentucky, Lexington
I think this paper is what Fauci Spotted:
Construction of in-frame chimeric plant viral genes by simplified PCR strategies.
Atreya CD, Atreya PL, Pirone TP. Plant Mol Biol. 1992 Jun;19
Site-directed mutations in the potyvirus HC-Pro gene affect helper component activity, virus accumulation, and symptom expression in infected tobacco plants.
Atreya CD, Atreya PL, Thornbury DW, Pirone TP.Virology. 1992 Nov
Mutational analysis of the coat protein N-terminal amino acids involved in potyvirus transmission by aphids.
Atreya PL, Lopez-Moya JJ, Chu M, Atreya CD, Pirone TP.J Gen Virol. 1995 Feb;76
…
Her papers then show a move to NIH
Affiliation: Laboratory of Infectious Diseases, National Institute of Allergy and Infectious Diseases, National Institutes of Health, Bethesda, Maryland 20892-0720, USA.
The NS1 protein of human respiratory syncytial virus is a potent inhibitor of minigenome transcription and RNA replication.
Atreya PL, Peeples ME, Collins PL.J Virol. 1998 Feb;
And then the move to FDA.
Affiliation: Laboratory of Pediatric and Respiratory Viral Diseases, DVP/CBER, Food and Drug Administration, Bethesda, MD 20892, USA.
Respiratory syncytial virus strain A2 is resistant to the antiviral effects of type I interferons and human MxA.
Atreya PL, Kulkarni S.Virology. 1999 Sep 1; (@ FDA)
Role of type I IFNs in the in vitro attenuation of live, temperature-sensitive vaccine strains of human respiratory syncytial virus.
Loveys DA, Kulkarni S, Atreya PL.Virology. 2000 Jun
……..
Her resume STINKS! She has three papers on human respiratory syncytial virus, and a bunch of early papers on cloning and tinkering with plants @ Univ Kentucky and earlier papers messing with fiber cell membranes of frog, chick, bovine, rabbit and human eye lenses @ Wayne State Univ, MI NOTHING ELSE except the Sex Card, Race Card and probably not American born.
……..
These are her picks:
CHAIR:
Hana El Sahly, M.D.
Baylor College of Medicine
May 18, 2020
Hana El Sahly on Remdesivir and the NIH’s Adaptive COVID-19 Treatment Trial (Well that answers WHO set up elders for DEATH!)
On May 15, Texas Monthly reported on their conversation with Dr. Hana El Sahly of Houston’s Baylor College of Medicine. In the coming days, she will begin registering hospitalized participants at Baylor St. Luke’s Medical Center and Ben Taub Hospital for the second phase of the National Institutes of Health’s Adaptive COVID-19 Treatment Trial, or ACTT. She’s Baylor’s lead investigator for participation in the program, which in its first phase analyzed a randomized, controlled trial designed to evaluate the safety and efficacy of the investigational antiviral remdesivir. Preliminary findings suggested that patients treated with remdesivir recovered faster than patients who received a placebo, which led to the May 1 announcement that remdesivir would be the first medication to receive FDA authorization for emergency use for COVID-19.
“We found that for patients who have COVID-19 pneumonia bad enough to get them to the hospital, treatment with remdesivir expedites the time to recovery by an average of four days per patient,” says El Sahly…
Hana El Sahly, M.D.
Education
Undergraduate education American University of Beirut, Lebannon Bachelor of Science, 1987-1990Medical education American University of Beirut, Lebannon School of Medicine, Doctor of Medicine, 1990-1994
Scientific Papers (46)
Several presentations on “HIV vaccines”
Fauci must love her:
Review Panels, Committees
Member, Safety Monitoring Committee, National Institutes of Health-sponsored vaccine clinical trial 05-0011; 2006
Reviewer, Loan Repayment Program, National Institute of Allergy and Infectious Diseases, National Institutes of Health; 2008
Member, Safety Monitoring Committee, National Institutes of Health-sponsored vaccine clinical trial 08-0009; 2009
Reviewer, Scientific Review Program, National Institute of Allergy and Infectious Diseases; 2010
Member, Data Safety Monitoring Board, Protein Sciences Corporation vaccine clinical trial PSC-22; 2010
Member, Safety Monitoring Committee, National Institutes of Health–sponsored study DMID 10-0043; 2011
Member, Safety Monitoring Committee, National Institutes of Health-sponsored study DMID 11-0007; 2011
Reviewer, Scientific Review Program, National Institutes of Health, P01 application “Towards A Universal Influenza Vaccine”; 2012
Member, Safety Monitoring Committee, National Institutes of Health sponsored study DMID 13-0087; 2014
Member, Publications Committee, Infectious Diseases Society of America; 2014-2017
Member, Safety Monitoring Committee, Mercia Pharma Inc-sponsored study NOVA MAS-1; 2015
Member of the Food and Drug Administration Vaccine and Related Biological Advisory Committee; 2016–
Reviewer, Influenza pre-applications, US Army Medical Research and Materiel Command-Congressionally Directed Medical Research Programs, 2016
WHAT THE HECK IS THIS!! => Member, ID week program planning committee, 2017-2019
Is this her daughter: Dr. Hana Mohammed Elsahly, MD 28, practicing in Houston, TX?
……
Paula Annunziato, M.D. ***
Vice President and Therapeutic Area Head
Vaccines Clinical Research
Merck
Seems to specialize in Pediatric Vaccines.
Nuv said…
…..
Archana Chatterjee, M.D., Ph.D.
Dean Chicago Medical School
Vice President for Medical Affairs
Rosalind Franklin University of Medicine and Science
M.B.B.S. (Equivalent to M.D.): Pune University, Maharashtra, India 1979-1983
Army Medical Corps, Military Hospital, Gaya, India, 1985 -1988
Major Scientific Interest: Vaccine development. She forgets to mention her main trial target is infants.
Principal Investigator: Recent Research Projects/Grants
GSK = GlaxoSmithKlinePled Guilty and Pay $3 Billion to Resolve Fraud Allegations & Failure to Report Safety Data – July 2012
(Nice people she worked for.)
Date: 2018-2019 Sponsor: Department of Health and Human Services, Administration For Community Living
Date: 2018-2020 Sponsor: Pfizer A Phase 2, Randomized,Trial ….Pneumococcal Conjugate Vaccine in Healthy Infants.
Date: 2018-2020 Sponsor: GSK …Study to Assess the Safety & Immunogenicity of Meningococcal Vaccine & 1 Pneumococcal Vaccine when Administered Concomitantly with Routine Vaccines to Healthy Infants.
Date: 2018-2020 Sponsor: GSK … dose-escalation study to evaluate safety, reactogenicity and immunogenicity of GSK Biologicals’ respiratory syncytial virus (RSV) investigational vaccine based on the RSV viral proteins F, N and M2-1 encoded by chimpanzee-derived adenovector.. when administered… to RSVseropositive infants aged 12 to 23 months.
Date: 2017-2019 Sponsor: MedImmune ….Study to Evaluate the Safety and Efficacy of MED18897, a Monoclonal Antibody With an Extended Half-life Against Respiratory Syncytial Virus, in Healthy Preterm Infants. [Are they going to give the infants the RSV?]
Date: 10/2015-10/2017 Sponsor: Merck…, Study to Evaluate the Safety, Tolerability, and Immunogenicity of Different Formulations of V114 [15-valent pneumococcal conjugate vaccine ] in Healthy Adults and Infants.
Date: 10/2015-10/2016 Sponsor: Astra Zeneca An observational study of RSV hospitalization in preterm infants.
Date: 9/2014-2017 Sponsor: GSK … multinational study … of GSK Biologicals’ MMR vaccine (209762)… compared to Merck (M-M-R®II or VaxPro), as a first dose, both co-administered with Varivax, Havrix (all subjects) and Prevnar 13 (US subset) in healthy children 12 to 15 months of age.
Peer-Reviewed Articles (120)
Appointments:
2020 – Invited to serve on NIH (NCI) Special Emphasis Panel to evaluate grant applications received in response to the RFA(s) with primary focus on HIV-Associated Malignancy Research
2019 to present – Invited to serve as and appointed a member on the Vaccines and Related Biological Products Advisory Committee (VRBPAC) of the United States Food and Drug Administration (US FDA)
2012 -2019 – Consultant to the US FDA
2014 – Merck Vision 2027 Expert Input Forum on Vaccine Policy
2008 – 2012 – Member, Anti Infective Drug Advisory Committee (AIDAC), Center for Drug Evaluation and Research, US FDA
2008 -2012 – Invited to serve on the National Vaccine Advisory Boards for Merck, GlaxoSmithKline and Novartis Pharmaceutical Companies
2008– Merck Vaccination Service Award, recognition of commitment to improving public health through vaccination.
2006 – Invited member, Sanofi-Pasteur, MedImmune, Abbott Pharmaceutical Companies’ National Advisory Boards
2003 – Invited Session Chair at an International Symposium organized by the Merieux Foundation entitled, “Vaccination in Tomorrow’s Society – New Information Pathways”. Annecy, France
Merieux Foundation …. AND THAT GETS INTERESTING…. WIKI
In October 2004, the FM was the beneficiary of a Franco-Chinese agreement that led to the creation of the Institut Pasteur de Shanghai.…
In 2012, the FM continued its partnership with the Chinese Academy of Medical Sciences.
In 2015, the CAMS-FM partnership founded the Christophe Mérieux Laboratory (CML) at the Institute of Pathogen Biology in Beijing to focus on the study pneumonia and tuberculosis. Researchers at the CML “benefit from and training modules developed by the Emerging Pathogens Laboratory in Lyon”,[5][6] a BSL-4 lab which was also built by the FM in 1999 and since 2005 is now operated by INSERM.[7]
In 2015, the FM participated in the donation by the French government of CIRI’s Biosafety Level 4 expertise to the Wuhan Institute of Virology.
In January 2017, a researcher who was financed by the CAMS-FM partnership participated in a study of human rhinovirus and genotype A21…..
https://en.wikipedia.org/wiki/Fondation_M%C3%A9rieux
WIKI
“Mentorship and sponsorship of faculty and learners has been a hallmark of Dr. Chatterjee’s entire thirty- year career in academic medicine…” LINK [I am sure she has been kissing FauXi’s ass for years to get funding.]
MORE:
….Board certified in general pediatrics and pediatric infectious diseases, she is nationally recognized for her work in vaccine development for human papilloma viruses and in antibiotic resistance. She has completed more than 100 clinical trials and published more than 50 peer-reviewed articles, 23 invited review articles, 17 book chapters and one book.
The first woman and person of color to serve as dean of CMS, Dr. Chatterjee, a native of India, earned her medical degree from the Armed Forces Medical College at Pune University in India and her PhD from the University of Nebraska Medical Center (UNMC) in Omaha….
https://www.rosalindfranklin.edu/news/rfu-announces-selection-of-new-dean-for-chicago-medical-school/
…..
CAPT Amanda Cohn, M.D.
Expertise: Pediatrics, Vaccines
Chief Medical Officer
National Center for Immunizations and Respiratory Diseases
Centers for Disease Control and Prevention
Immunization and Respiratory Diseases (NCIRD) MISSION | CDC
The mission of the National Center for Immunization and Respiratory Diseases (NCIRD) is the prevention of disease, disability, and death through immunization and by control of respiratory and related diseases.
CDC
POSTGRADUATE TRAINING 2004 – 2006 Epidemic Intelligence Service, CDC, Atlanta, GA
WORK EXPERIENCE :
3/2019-present Chief Medical Officer (Acting), Vaccine Policy, Preparedness, and Global Health, Office of the Director, NCIRD, CDC
11/2015-present Executive Secretary, ACIP and Senior Advisor, Vaccines Office of the Director, NCIRD, CDC
5/2014-11/2015 Deputy Division Director, Immunization Services Division, NCIRD, CDC
01/2013-05/2014 Acting Epidemiology Team Lead Meningitis and Vaccine Preventable Diseases, DBD, NCIRD, OD
06/2006-12/2012 Medical Officer, Epidemiology Team Meningitis and Vaccine Preventable Diseases, Division of Bacterial Disease, NCIRD, CDC
07/2004-06/2006 Epidemic Intelligence Service (EIS) Officer, Epidemiology Program Office Centers for Disease Control and Prevention, Atlanta, GA Assigned to: Bacterial Vaccine Preventable Diseases Branch, National Immunization Program
Scientific papers: 59 many authored with Nancy E Messonnier
Great titles like:
- Multistate Outbreak of Respiratory Infections Among Unaccompanied Children, June 2014-July 2014.
Conclusions: This respiratory disease outbreak was due to multiple pathogens, including Streptococcus pneumoniae serotype 5 and influenza viruses. Pneumococcal and influenza vaccinations prevented further transmission. Future efforts to prevent similar outbreaks will benefit from use of both vaccines. https://pubmed.ncbi.nlm.nih.gov/27001799/
[How about CLOSING THE DARN BORDERS!] - Understanding Factors Affecting University A Students’ Decision to Receive an Unlicensed Serogroup B Meningococcal Vaccine.
- Compliance with recommendations and opportunities for vaccination at ages 11 to 12 years: evaluation of the 2009 national immunization survey-teen.
- Adolescent immunizations and other clinical preventive services: a needle and a hook?
- Immunizations in the United States: a rite of passage.
- Attitudes, practices, and preferences of pediatricians regarding initiation of hepatitis B immunization at birth.
……
Hayley Gans, M.D.
Expertise: Pediatrics, Infectious Diseases
Professor of Pediatrics
Department of Pediatrics
Stanford University Medical Center
Fellowship: Stanford University School of Medicine (1998) CA
- Medical Education: State University of New York Syracuse Medical School Registrar (1991) NY
- Board Certification: American Board of Pediatrics, Pediatric Infectious Diseases (1999)
- Residency: Stanford University Medical Center (1994) CA
- Internship: Stanford University Medical Center (1992) CA
- M.D., SUNY at Syracuse, Medicine (1991)
Fellowship Program Director, Pediatric Infectious Diseases (2006 – 2017)
- Co-director, Pediatric Infectious Diseases Program for Immunocompromised Hosts, Children’s Hospital at Stanford (2013 – Present)
- Associate Fellowship Director, Pediatric Infectious Diseases, Stanford University Medical Center (2017 – Present)
- Director, Fellowship Education, Department of Pediatrics, Stanford University Medical Center (2017 – Present)
Sort of BLAAaaaah until you look at this paper:
Remember her focus is kids.
July 2020 Lancet preprint.
Kinetics of SARS-CoV-2 Positivity of Infected and Recovered Patients: A Single Center COVID-19 Experience and Potential Implications https://autopapers.ssrn.com/sol3/papers.cfm?abstract_id=3605268
Jia Huang – Southern University of Science and Technology CHINA and 42 other authors with only 7 others not Chinese. The other universities were Sichuan University, China and Sanford.
https://autopapers.ssrn.com/sol3/cf_dev/AbsByAuth.cfm?per_id=4257785
And then it REALLY GETS GOOD:
FUNDING STATEMENT: This work was supported by grants from Sanming Project of Medicine in Shenzhen (Jia Huang, No. SZSM201812065); Bill & Melinda Gates Foundations (Lei Liu); and from National Natural Science Foundation of China (Jia Huang, No. 81501651)
DECLARATION OF INTERESTS: The authors declare no competing interests.
ETHICS APPROVAL STATEMENT: This study was approved by the Ethics Committee of the Second Affiliated Hospital of Southern University of Science and Technology.[CHINA]
Abstract
BACKGROUND: Recurrence of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) positive detection in infected but recovered individuals has been reported. Patients who have recovered from coronavirus disease 2019 (COVID-19) could profoundly impact the health care system if a subset were to be polymerase chain reaction (PCR)-positive again with reactivated SARS-CoV-2. We sought to define the kinetics and relevance of PCR-positive recurrence during recovery from acute COVID-19 to better understand risks for prolonged infectivity and reinfection.
METHODS: A series of COVID-19 414 patients, at The Second Affiliated Hospital of Southern University of Science and Technology in Shenzhen, China from January 11 to April 23, 2020. Univariable and multivariable statistical analysis of inpatient data were performed to develop an algorithm to predict patients at risk of recurrence of PCR positivity.
[REMEMBER PCR TESTS RETURN MAJOR FALSE POSITIVES – Reiner Fuellmich say this guy Droston isn’t a Doctor at all, but a bull**** artist. Christian Drosten & the Fraud Behind COVID 19 PCR Testing ]
FINDINGS: 16·7% recovered patients with PCR positive recurring one to three times, despite being in strict quarantine. Younger patients with mild pulmonary respiratory syndrome had higher risk of PCR positivity recurrence. The recurrence prediction model had an area under the ROC curve of 0·786.
INTERPRETATION: This case series provides clinical characteristics of recovered COVID-19 patients with recurrent SARS-CoV-2 positivity, despite strict quarantine, at a 16·7% rate. Use of a recurrence prediction algorithm may identify patients at high risk of recurrent SARS-CoV-2 positivity and help understand reactivation and reinfection possibilities to establish protocols for health policy.
LANCET
This is a very important paper because it REFUTES NATURAL IMMUNITY and green lights MORE DRACONIAN ECONOMY KILLING ‘Health Measures’
……
Holly Janes, Ph.D.
Expertise: Biostatistics
Professor — Fred Hutchinson Cancer Research Center
Vaccine and Infectious Disease Division
Division of Public Health Sciences – Seattle, WA
Dr. Holly Janes is a biostatistician working on the design and analysis of vaccine studies, with a particular expertise in HIV prevention and vaccine science. She also develops and applies statistical methodology for evaluating biomarkers for risk prediction and optimizing treatment decisions
Current Projects
Leadership for the Statistical Data Management Center of the HIV Vaccine Trials Network
Statistical methods for HIV prevention efficacy trials
Statistical methods for human challenge studies
Statistical evaluation of biomarkers for making treatment decisions https://www.fredhutch.org/en/faculty-lab-directory/janes-holly.html
HONORS, AWARDS, SCHOLARSHIPS:
2008 Travel Award, AIDS Vaccine Conference, Global HIV Vaccine Enterprise
2000 Cardiovascular Biostatistics Training Grant, National Institutes of Health
EDITORIAL RESPONSIBILITIES:
Associate Editor Journal of the National Cancer Institute (2015-2018)
Diagnostic and Prognostic Research (2016-present)
Statistical Communications in Infectious Diseases (2019-present)
RESEARCH FUNDING:
Active Funding:
2 UM1 AI068635 (PI: Gilbert P) 01/01/2014 – 11/30/2020 5.4 Calendar NIH/NIAID SDMC HIV Vaccine Trials Network
2 R01 CA152089 (PI: Janes H) 07/01/2010 – 11/30/2021 4.8 Calendar
NIH/NCI
Statistical Methods for Evaluating Markers for Treatment Selection
Interventions for disease treatment and prevention can potentially be made more cost-effective by using markers to identify in advance the individuals most likely to benefit from the treatment, and thus avoid treating those unlikely to benefit. [Rationed Health Care anyone?]
Lots more Mostly NIH and then this goodie:
38744 7/1/2006-4/30/2012
Bill & Melinda Gates Foundation
Vaccine Immunology Statistical Center (VISC) The VISC will provide 1) statistical and study design support for pre-clinical vaccine performance trials, 2) centralized data management services for the standardized evaluation of vaccine candidates, 3) development of new statistical methods for cross-species correlates-of-protection analysis.
Role: Faculty Statistician
BIBLIOGRAPHY Publications in Refereed Journals
1. Pepe MS, Janes H, Longton G, Leisenring W, Newcomb P. Limitations of the odds ratio in gauging the performance of a diagnostic, prognostic, or screening marker. Am J Epidemiol. 2004;159(9):882-90.
2. Janes H, Pepe M, Kooperberg C, Newcomb P. Identifying target populations for screening or not screening using logic regression. Stat Med. 2005;24(9):1321-38.
.
.
12. McElrath MJ, De Rosa SC, Moodie Z, Dubey S, Kierstead L, Janes H, Defawe OD, Carter DK, Hural J, Akondy R, Buchbinder SP, Robertson MN, Mehrotra DV, Self SG, Corey L, Shiver JW, Casimiro DR. HIV-1 vaccine-induced immunity in the test-of-concept Step study: A casecohort analysis. Lancet. 2008;372(9653):1894-905. PMCID: 2774110.
13. Pepe MS, Feng Z, Janes H, Bossuyt PM, Potter JD. Pivotal evaluation of the accuracy of a biomarker used for classification or prediction: Standards for study design. J Natl Cancer Inst. 2008;100(20):1432-8. PMCID: 2567415.
.
.
21. Barnabas RV, Wasserheit JN, Huang Y, Janes H, Morrow R, Fuchs J, Mark KE, Casapia M, Mehrotra DV, Buchbinder SP, Corey L. Impact of herpes simplex virus type 2 on HIV-1 acquisition and progression in an HIV vaccine trial (the Step study). J Acquir Immune Defic Syndr. 2011;57(3):238-44. PMCID: 3446850.
22. Fitzgerald DW, Janes H, Robertson M, Coombs R, Frank I, Gilbert P, Loufty M, Mehrotra D, Duerr A. An Ad5-vectored HIV-1 vaccine elicits cell-mediated immunity but does not affect disease progression in HIV-1-infected male subjects: Results from a randomized placebo-controlled trial (the Step study). J Infect Dis. 2011;203(6):765-72. PMCID: 3119328.
And many more. I am sure Fauci loves her.
……
Michael Kurilla, M.D., Ph.D.
Expertise: Infectious Diseases, Pathology
Director, Division of Clinical Innovation
National Center for Advancing Translation Sciences
National Institutes of Health
The National Center for Advancing Translational Sciences (NCATS) is one of 27 Institutes and Centers at the National Institutes of Health (NIH). The focus of NCATS is to advance the science of translation, which is the process of turning observations into interventions to improve health.
National Center for Advancing Translation Sciences
……
Myron Levine, M.D., D.T.P.H., F.A.A.P
Expertise: Infectious Diseases
Simon & Bessie Grollman Distinguished Professor
Associate Dean for Global Health
Vaccinology and Infectious Diseases Center for Vaccine Development
University of Maryland School of Medicine
University of Maryland School of Medicine
For more than a year, researchers at the Center for Vaccine Development and Global Health (CVD) at the University of Maryland School of Medicine (UMSOM) have been working tirelessly on COVID-19 research, helping to pave the way for the use of vaccines and therapies that are being administered across the country.
Under the leadership of CVD director Kathleen Neuzil, MD, MPH, FIDSA, the Myron M. Levine, MD, DTPH, Professor in Vaccinology at UMSOM, researchers quickly pivoted decades of vaccine and infectious disease research experience toward combating this deadly virus, which continues to impact millions of people around the world.
Faculty at CVD have served in critical leadership roles in U.S. and international research and policy efforts. For example, Neuzil co-chaired the COVID-19 Prevention Trials Network, a research network established by the National Institute of Allergy and Infectious Diseases [NIAID Dr. Fauciwas appointed director of NIAID in 1984.] in response to the pandemic. Vaccine research at CVD continues, with an emphasis on reaching the populations most impacted by COVID-19 and testing pediatric vaccines.
CVD experts have launched an expansive grassroots campaign to educate the community and reach those who have been hit the hardest by this terrible virus, including members of the Black and Brown communities, the elderly, and those with underlying health risks.
“Our CVD team has worked tirelessly and meticulously to advance COVID-19 vaccines and to ensure they are reaching the most affected populations,” Neuzil said. “Our work continues as we begin testing vaccines in children and investigate booster vaccines to address the risk of COVID-19 variants.” [Like this Dude is neutral?]
Our research, surveillance and vaccine development focuses on four key areas: Enteric Diseases, Malaria, Influenza and Respiratory Diseases, and Emerging Pathogens.
Overview
Our faculty and staff are experts in the field of global health and vaccinology, and they are dedicated to improving global health by conducting innovative, world-leading research in Baltimore and around the world. Our key mission is to harness the power of vaccines to prevent disease and save lives in the most vulnerable populations.
…….
H. Cody Meissner, M.D.
Expertise: Infectious Diseases
Professor of Pediatrics
Tufts University School of Medicine
Director, Pediatric Infectious Disease
Tufts Medical Center
POST GRADUATE TRAINING
1973 – 1975 Internship and Residency Boston Floating Hospital New England Medical Center Boston, MA
1975 – 1977 Research Associate Public Health Service National Institute of Child Health and Human Development National Institute (NICHD) Bethesda, MD Parent Agency is National Institutes of Health (Fauci)
2008 – Present Advisory Committee on Immunization Practices (ACIP) Centers for Disease Control and Prevention (CDC)
2010 – Present Massachusetts Vaccine Purchasing Advisory Council
2017 – Present National Vaccine Advisory Committee, United States Department of Health and Human Services
2017 – Present Vaccine Injury Compensation Program, United States Department of Health and Human Services
AWARDS
Massachusetts 2017 Recipient of the CDC Childhood Immunization Award
The National Vaccine Injury Compensation Program: Striking a Balance Between Individual Rights and Community Benefit.
Meissner HC, Nair N, Plotkin SA. JAMA. 2019 Jan 29
The Importance of MMR Immunization in the United States.
Perrone O, Meissner HC. Pediatrics. 2020 Aug
Principles of Vaccine Licensure, Approval, and Recommendations for Use.
Pickering LK, Meissner HC, Orenstein WA, Cohn AC. Mayo Clin Proc. Epub 2020 Feb 13.
H. Cody Meissner, MD, is a leading national expert on childhood vaccinations who consults with the Centers for Disease Control and Prevention on periodic updates to the recommended immunization schedule for newborns through 18-year-olds. At Tufts Children’s Hospital at Tufts Medical Center he heads the Division of Pediatric Infectious Diseases
….
Paul Offit, M.D.
Expertise: Infectious Diseases
Professor of Pediatrics
Division of Infectious Diseases
The Children’s Hospital of Philadelphia
Paul A. Offit, MD is the Director of the Vaccine Education Center at the Children’s Hospital of Philadelphia as well as the Maurice R. Hilleman Professor of Vaccinology and a Professor of Pediatrics at the Perelman School of Medicine at the University of Pennsylvania. He is a recipient of many awards including the J. Edmund Bradley Prize for Excellence in Pediatrics from the University of Maryland Medical School, the Young Investigator Award in Vaccine Development from the Infectious Disease Society of America, and a Research Career Development Award from the National Institutes of Health. Dr. Offit has published more than 160 papers in medical and scientific journals in the areas of rotavirus-specific immune responses and vaccine safety. He is also the coinventor of the rotavirus vaccine, RotaTeq, recommended for universal use in infants by the CDC….
FDA
In 2017, Dr. Offit was a weekly columnist for The Daily Beast.
Papers:
2018
Plotkin, S.A., P.A. Offit, and P. Bégué, : “Vaccine mandates in France will save lives,” Science 359: 283-284, 2018.
Plotkin SA, Offit PA, Reiss D.: Important New Resource for Clinicians Giving Expert Witness Testimony on Vaccines. Pediatr Infect Dis J. 37(12), Dec. 2018.
To the Editors:
Vaccination is under attack by individuals who occasionally use the legal system to oppose mandatory vaccination laws and in some cases to obtain exemptions for particular children whose parents are opposed to vaccination. During the legal proceedings, as we have witnessed, experts testifying in favor of vaccination may be challenged with references from journals of doubtful quality that oppose vaccination.
To provide important references that discuss and disprove claims made against vaccines, the Vaccine Education Center at the Children’s Hospital of Philadelphia has created a library of references addressing certain safety issues that may be useful as an aid and refresher to clinicians giving expert testimony on the safety of vaccines and to lawyers defending vaccination of children.
The Children’s Hospital of Philadelphia legal library may be entered through the web address vaccine.chop.edu/safety-references.
We would be grateful if you could inform your colleagues about the availability of this resource, which should be of great value for experts testifying for vaccination and for clinicians who need to convince parents about vaccine safety. https://journals.lww.com/pidj/Fulltext/2018/12000/Important_New_Resource_for_Clinicians_Giving.42.aspx
2017
Offit, P.A.: “Commentary: Science Denialism Isn’t New to the Trump Administration,” Philadelphia Inquirer December 22 2017.
Offit, P.A.: “By Regulating Homeopathic Remedies, FDA Holds ‘Modern-Day Snake-Oil Salesmen’ Accountable,“ Philadelphia Inquirer December 28 2017.
2013
A look at his recent papers shows he is targeting vaccine hesitant parents.
https://pubmed.ncbi.nlm.nih.gov/?term=Offit+PA&cauthor_id=24011750&size=20
…..
Steven Pergam, M.D.
Expertise: Infectious Diseases
Medical Director
Infection Prevention
Seattle Cancer Care Alliance — Seattle, WA
He seems to specialize in cancer and immuno-compromised and seems to be the best of a bad bunch. But then we look at this:
SPECIAL NATIONAL RESPONSIBILITIES:
2009–2010 Independent Safety Monitor, NIH/NIAD, DMID Influenza Protocols: 09-0039, 09-0043, 09-0047, 09-0053, and 09-0054: H1N1
2010–2011 Independent Safety Monitor, NIH/NIAID, DMID Protocol 09-0002: Comparison of the Safety and Immunogenicity of Lyophilized IMVAMUNE® (1×108 TCID50) versus Liquid Formulation IMVAMUNE® (1×108 TCID50) Administered by the Subcutaneous Route and a Lower Dose Liquid Formulation IMVAMUNE® (2×107 TCID50) Administered by the Intradermal Route in Healthy Vaccinia-Naïve Individuals (Bavarian Nordic)
2011–2013 Member, Data and Safety Monitoring Board: Effect of tenofovir on genital HSV shedding: a randomized, double-blind, placebo-controlled, clinical trial
2015-present Member, Zoster Working Group, Advisory Committee on Immunization Practices (ACIP), Centers for Disease Control and Prevention, Department of Health and Human Services
2016-present Member, Abstract Selection Committee, Association for Professionals in Infection Control and Epidemiology (APIC)
2016-2017 Independent Safety Monitor, NIH/NAID, DMID Protocol 16-0117: Comparison .of High vs. Standard Dose Flu Vaccine in Pediatric Stem Cell Transplant Recipients
.
.
.
15. RESEARCH FUNDING
Current: Washington Vaccine Alliance (WAVA) Pilot Award (PI: S. Pergam) 10/1/13-6/30/20 Interactions between gastrointestinal microbiota, Influenza vaccine responses and respiratory viral infections in a large cohort of clinic employees
BAA-NIAID [Fauxi Director]-DMID-NIH-AI (PI: M. Ison; Subcontract PI: Pergam) 5/1/16-4/30/20 Phase II Multi-Center, Prospective, Randomized, Double-Blind Study of Nitazoxanide in Acute and Chronic Norovirus in Hematopoietic Stem Cell and Solid Organ Transplant Recipients 1U01AI132004-NIAID (PI: N.Halasa; Subcontract PI: Pergam)
7/5/2017-6/30/20 High vs. Standard Dose Flu Vaccine in Adult Stem Cell Transplant Recipients 1R01AI134808-NIAID (PI: D. Fredricks)
.
.
Completed:
NIH/NIAID T32 AI007-044 (PI: W. Stamm) 9/1/05-2/1/07 Host Defense Training in Allergy and Infectious Diseases
.
.
Industry Sponsored Clinical Trials:
Chimerix, Inc. (PI: Pergam) 2016-current An Intermediate-size, Expanded Access Protocol to Provide Bincidofovir for the Treatment of Serious Adenovirus Infection or Disease, Protocol CMX001-35”
6/17/2017-present Prior Industry Trials Merck, Sharp & Dohme Co., Inc (PI: Pergam)
2012-2015 Pergam, SA – CV Page 15 A Phase III, Double-Blind, Randomized, Placebo-Controlled, Multicenter Clinical Trial to Study the Safety, Tolerability, Efficacy, and Immunogenicity of V212 in Recipients of Autologous Hematopoietic Cell Transplants (HCT)
Cubist Pharmaceuticals, Inc.* (PI: Pergam) 2013-2015 A Phase IIIb, Multi-Center, Double-Blind, Randomized, Placebo-Controlled Study to Demonstrate the Safety & Efficacy of Fidaxomicin for Prophylaxis against C difficile-Associated Diarrhea in Individuals Undergoing Hematopoietic Cell Transplants (HCT) *formerly Optimer pharmaceuticals
KRT16/26/21, 01:40 PM
$ACXP In December 2014, Merck ($MRK) paid US$9.5 billion for Cubist ($CBST) largely to obtain marketing access to agents daptomycin and fidaxomicin. https://stocktwits.com/symbol/CBST
Chimerix, Inc. (PI: Pergam) 2016 A Multicenter Non-Interventional Study to Obtain Retrospective Data for Subjects Previously Diagnosed with Adenovirus Infection to serve as Matched Historical Controls for Study CMX001-304; Protocol No. CMX001-305
Chimerix, Inc. (PI: Pergam) 2015 – 2017 A Phase 3, Open-label, Multicenter Study of the Safety/Tolerability and Efficacy of Brincidofovir (CMX001) for the Prevention of Adenovirus (AdV) Disease in Subjects with Asymptomatic AdV Infection at Risk of Progression and for the Treatment of Subjects with Localized or Disseminated AdV Disease
Chimerix, Inc.
All that shimmers isn’t … enhanced by lipid conjugate technology. Chimerix is a development-stage biopharmaceutical company, dedicated to accelerating the advancement of innovative for patients living with cancer and other serious diseases. Its two clinical-stage development programs include dociparstat sodium (DSTAT) and brincidofovir (BCV). DSTAT, is a glycosaminoglycan derivative of heparin with known anti-inflammatory properties and BCV is an oral antiviral in development for the treatment of smallpox.
2505 Meridian Pkwy Ste 100 Durham, NC,
https://www.dnb.com/business-directory/company-profiles.chimerix_inc.a1878daaef1b59d25a1d2e8876c4b4bf.html
Chimerix, Inc.’s key principal is Michael A Sherman. Chimerix, Inc. has 54 employees
https://wallmine.com/people/8557/michael-a-sherman
…..
Jay Portnoy, M.D.
Expertise: Consumer Representative (This the guy who is supposed to represent the interests of the Public.)
Professor of Pediatrics
Medical Director of Telemedicine Section of Allergy, Asthma and Immunology
Children’s Mercy Hospital Kansas City, MO
Offices and Board of Directors:
American Board of Allergy & Immunology (ABAI). 2014-present.
Vice President, American College of Allergy, Asthma & Immunology 2005-6.
Board of Directors, Black Healthcare Coalition. 2006-2009. [He is WHITE]
Editorships and Editorial Boards
Regional Editor, World Allergy Organization Journal. 2008 to 2012.
Section Editor, Annals of Allergy and Asthma. Appointed 2002 to 2005
Editor, Current Opinion on Allergy & Asthma. Issue on Pediatric Allergy. 2004 and 2005
Editor, Current Allergy and Asthma Reports. Issue on Pediatric Allergy. 2001-2013
Editorial Board, Allergy Watch. 1998-2001.
Editorial Board, Annals of Allergy and Asthma. 1994 to 2006
Editorial Board, Current Allergy Practice. 1993 to present
Other Appointments
FDA advisory panel (CBER), Allergenic Extracts.
2017-present FDA advisory panel (CDER). Respiratory and allergy drugs.
2010-present FDA advisory panel (CBER), Allergenic Extracts.
2005-2010 Special Emphasis Panel. T-cell Epitopes. NIAID. 2011.
https://www.fda.gov/media/105541/download
The guy has 151 papers mainly dealing with allergy so I am not going to look at all of them.
He seems to work with Environmental Allergens Workgroup. Also with American College of Allergy, Asthma & Immunology – “…a professional medical association of more than 6,000 allergist-immunologists and allied health professionals…” He is a Fellow, American Academy of Allergy, Asthma, and Immunology (AAAAI) …. if you search long enough…. You find an AAAAI Legislative Action article urging Allergists to support Fauci’s funding.
NIAID, NIEHS, NHLBI, MCAN Workshop Report: The Indoor Environment and Childhood Asthma: Implications for Home Environmental Intervention in Asthma Prevention and Management
The National Institute of Allergy and Infectious Diseases (NIAID), National Institute of Environmental Health Sciences (NIEHS), National Heart, Lung, and Blood Institute (NHLBI), and Merck Childhood Asthma Network (MCAN) sponsored a joint workshop to discuss the current state of the science with respect to the indoor environment and its effects…
Adverse reactions to vaccines practice parameter 2012 update
…..Thus although patients with a history of mild reactions to egg ingestion (hives only) can receive their vaccine in a primary care provider’s office, those with a history of more severe reactions (cardiovascular, respiratory, or gastrointestinal symptoms) should receive the influenza vaccine in an allergist’s office. In both cases, personnel to recognize and equipment to treat anaphylaxis need to be immediately available, but the allergist’s office affords additional expertise in this area should it be required…..
…..There has been a great deal of additional information published over the past year demonstrating the safety of influenza vaccination in patients with egg allergy. Health care providers should no longer withhold the vaccine from any patient with egg allergy. In an update to recommendations made in the last year, it is now considered safe for patients even with a history of a severe egg allergy to receive influenza vaccination…..
No worries, we will revive you when you almost die of anaphylaxtic shock, it is utmost importance for us to jab you with a shot that is probably useless so we can get paid our bonus.
…..
Andrea Shane, M.D., M.P.H., M.Sc.
Expertise: Pediatric & Infectious Diseases
Professor of Pediatrics
Director Division of Pediatric Infectious Diseases
Emory University School of Medicine – Atlanta, GA
Joint appointment:
Assistant Professor of Global Health Hubert Department of Global Health, Rollins School of Public Health, Emory University 01 September 2013-present
Military or Government Service: Lieutenant Commander, United States Public Health Service, 2001-2003; Inactive Reserve Corps (IRC) 2003-until IRC dissolved in 2010.
……
The United States Public Health Service is a collection of agencies of the Department of Health and Human Services concerned with public health, containing eight out of the department’s eleven operating divisions. The Assistant Secretary for Health oversees the PHS.
WIKI
ALSO:
OASH oversees the Department’s key public health offices and programs, a number of Presidential and Secretarial advisory committees, 10 regional health offices across the nation, and the Office of the Surgeon General and the U.S. Public Health Service Commissioned Corps. https://www.hhs.gov/ash/index.html
……
Centers for Disease Control and Prevention, Advisory Committee on Immunization Practices (ACIP) respiratory syncytial virus (RSV) immunoprophylaxis working group, appointed member, 2009-until committee dissolved by CDC in 2011.
Infectious Diseases Society of America (IDSA) National Global Public Health Committee (NGPHC), appointed member 2010-2013.
World Society of Pediatric Infectious Diseases (WSPID), Board Member and member of the Education Committee representing the Pediatric Infectious Disease Society (PIDS), appointed 2017; term through 2019.
[THIS IS WHERE SHE HAS A LOT OF POWER]
Manuscript reviewer:
American Journal of Infection Control, 2001-2003
Clinical Infectious Disease Journal, 2003-present
Journal of Infectious Diseases, 2003 – present
Pediatrics, 2006 – present
Journal of Pediatrics, 2006-present
The Pediatric Infectious Disease Journal, 2003-present
Infection Control and Hospital Epidemiology, 2003 – present
Archives of Pediatrics and Adolescent Medicine, 2006 – present
Emerging Infectious Diseases Journal, 2006 – present
Neonatology, 2008 – 2010
Journal of American Medical Association, 2009 – present
JAMA Pediatrics, 2013 – present
Journal of Pediatric Infectious Diseases, 2013-present
Pediatric Research 2017-present
Clinical Therapeutics, 2017-present
Faculty of 1000 (f1000), Public Health and Epidemiology section, post publication peer review of publications, 2009 -2011. [WTF???]
Pediatric Infectious Disease section with creation of the section, 2011-2014.
Honors and Awards:
International exchange fellowship, Children’s Hospital at Montefiore and Beijing Children’s Hospital, Beijing, China October-November, 1999
Department of Health and Human Services, Public Health Service Crisis Response Service Award, 2002
Department of Health and Human Services, Public Health Service Outstanding Unit Citation, 2002
National Foundation for Infectious Diseases (NFID) Advanced Vaccinology Course Travel Grant to attend ADVAC 9, Annecy, France, 2008
National Institute of Allergy and Infectious Diseases, Division of Microbiology and Infectious Diseases, Special Recognition, H1N1 influenza research, 2010
Center for Disease Control and Prevention, National Center for Emerging and Zoonotic Infectious Diseases Award for Excellence in Partnering-Domestic to NETEC (the National Ebola Training and Education Center)…. This award recognizes programs’ initiative and effectiveness through establishing and sustaining a strategic partnership with government, private sector, volunteer, or nonprofit organizations, 24 March 2016.
Contracts:
Co- investigator, NIH/NIAID/DMID Vaccine and Treatment Evaluation Unit (VTEU) – Emory University School of Medicine. Role: Site PI on rotavirus vaccine cross-over trial, DMID #08- 0017 and influenza vaccine to breastfeeding women trial, DMID#09-007; site co- investigator on other trials. Salary support, 01 August 2007- 01 August 2016….
………….
Paul Spearman, M.D.
Expertise: Pediatric & Infectious Diseases
Director, Division of Infectious Diseases
Albert B. Sabin Chair in Pediatric Infectious Diseases
Cincinnati Children’s Hospital Medical Center
Professor, Department of Pediatrics
University of Cincinnati School of Medicine Cincinnati, OH
This guy is a really big heavy weight. There is cross-over with the lady, Andrea Shane above. Any bets he pulled her in to be his ‘female puppet’ – a good little government soldier?
11/2005-09/2016: Professor and Division Director Nahmias-Schinazi Research Chair Pediatric Infectious Diseases Department of Pediatrics Emory University School of Medicine
11/2005-09/2016: Associate Director for Pediatric Studies Emory Vaccine Center Atlanta, GA
03/2009-09/2016: Vice Chair for Research Department of Pediatrics Emory University School of Medicine
03/2009-09/2016: Chief Research Officer Children’s Healthcare of Atlanta Atlanta, GA
[Andrea L. Shane is Attending Pediatrician Children’s Healthcare of Atlanta Emory Healthcare Grady Health 01 August 2006 – present ]
This guy has a full page of
COMMITTEE MEMBERSHIPS:
a. National and International:
NIH Councils and Study Sections Chair, NIH ZRG1 AARR-E (41)
December 2016 Member, NIH ZRG1 AARR-P (02)
December 2016 Chair, NIH SEP: ZRG1 AARR-K (02)M; AIDS and related research SEP
August 2016 Chair, NCI Board of Scientific Counselors, Site Visit Team, Review of HIV DRP, Frederick, MD
July 2016 Chair, NIH SEP: ZDE1; Approaches to Eliminate HIV and Opportunistic Pathogens from Oral Reservoirs
November 2015 Chair, NIH SEP: ZRG1 AARR-E; AIDS and AIDS-related
July 2015 Chair, NIH SEP: Basic Research on HIV Persistence
March 2015 Chair, NIH/NIDCR Review Panel on HIV and Oral
March 2015 Opportunistic Pathogens
.
.
.
NIH/NIAID HIV Vaccine Trials Network
Protocol Chair, HVTN 088 Protocol 2010-present
Chair, Chiron/Novartis Products Development Team 2000-2007
Chair, Wyeth Products Development Team 2001-2007 Protocol
Chair, HVTN 049 Protocol 2002-2007 Protocol
Chair, HVTN 056 Protocol 2002-2006 Protocol
Chair, HVTN 061 Protocol 2003-2005 Member, HVTN Phase I-II Committee 2002-2005 Protocol
Chair, HVTN 088 Protocol 2010-present
NIH/NIAID/DMID Vaccine and Treatment Evaluation Unit Co-Principal Investigator, Emory VTEU site 2007-present
Protocol Chair, VTEU 0008 Protocol 2009-2014
NIH/NICHD-Westat/NIAID IMPAACT Network Principal Investigator, Emory IMPAACT site 2014-present
.
.
.
CONSULTANTSHIPS:
Chiron, HIV Vaccines Development Team, Emeryville, CA 2003, 2004
Wyeth, HIV Vaccine Programs, Pearl River, NY 2003, 2004
EDITORSHIPS AND EDITORIAL BOARDS: [Again this is where a lot of power lies.]
Member of Editorial Board, Journal of Virology
Member of Editorial Board, Virology
Member of Editorial Board, Current HIV Research
Academic Editor, PLoS One
MANUSCRIPT REVIEWER: [There is that POWER again]
Journal of Virology (numerous, 1995-present)
Virology (numerous, 1998-present)
Current HIV Research (2001-present)
Ad Hoc reviewer, Biochemistry (2005, 2006)
Ad Hoc reviewer, Traffic (2005, 2006, 2007, 2013)
Ad Hoc reviewer, JAIDS (2004, 2011, 2012, 2013, 2014, 2015)
Ad Hoc reviewer, JBC (1997-present)
Ad Hoc reviewer, Leukocyte Biol (2000)
Ad Hoc reviewer, Vaccine (2000-2016)
Ad Hoc reviewer, Virus Research (2005, 2012, 2012, 2013)
Ad Hoc reviewer, Nature Structural Biology (2005)
Ad Hoc reviewer, PLOs Medicine (2006, 2007, 2008)
Ad Hoc reviewer, J Mol Biol (2007,2012, 2015, 2016)
Ad Hoc reviewer, PNAS (2007, 2008, 2009,2012, 2013, 2014)
Ad Hoc reviewer, JCB (2007, 2008, 2010, 2011,2012, 2013)
Ad Hoc reviewer, PLOs One (2008, 2009, 2010,2011,2012, 2013, 2014) 6
Ad Hoc reviewer, Cell Host and Microbe (2008-present)
Ad Hoc reviewer, Nature Medicine (2009, 2011,2012, 2016)
Ad Hoc reviewer, PLOs Pathogens (2009-present) Ad Hoc reviewer, J Immunology (2010, 2011, 2013) Ad Hoc reviewer, Retrovirology (2011-present)
.
.
GRANT SUPPORT:
a. Active Support
- Federally funded:
NIH R01 AI058828: Role of Vpu in HIV Particle Assembly. Funded since 2004, currently in no-cost extension with competing renewal under review.
NIH R01 GM111027-17A1: Viral and Cellular Determinants of HIV-1 Assembly. Funded 9/16/2013-8/31/2017 (Principal Investigator). $200,000 initial period; $800,000 direct costs.
NIH R01AI11863: Mucosal Protection against HIV Generated by PIV5 Priming and VLP Boosting. Funded 4/01/2014-8/31/2018 (Principal Investigator, Multiple PI grant). $351,366/yr. - Private foundation funded:
None presently. - Industry Contracts:
None presently
b. Previous Support:
NIH R01HL125042: HIV-induced Redox Stress and the Alveolar Macrophage as a Resistant Reservoir. Funded 7/01/2014-6/30/2018 (Principal Investigator, Multiple PI grant). $686,584/yr; relinquished upon relocation to Cincinnati.
NIH K12 HD072245: Atlanta Pediatric Scholars Program. Funded 04/01/2011-11/30/2016 (Program Director). $324,000/yr.
HHSN275201300003C: Westat/NICHD Contract- IMPAACT Network; Pediatric and Adolescent HIV/AIDS Research Program at Emory University. Funded 9/01/2014-8/31/2019 (Site Principal Investigator). $450,000/yr estimated.
NIAID-DMID-NIH AI2012144: Vaccine and Treatment Evaluation Units (VTEU). Funded 9/13/2013-9/12/2020. (Co-Principal Investigator). $4-5M/yr estimated. 8
NIH R21 AI098592: HIV-specific B cell repertoire in humans following cross-clade immunization. Funded 7/01/2012-6/30/2014 (Principal Investigator). $150,000 initial period; $275,000 direct costs.
NIH R01 AI090656: Broadly-reactive antibodies against chimeric virus-host antigens. Funded 06/14/2010-05/31/2014 (Co-investigator).
I wonder if he knows Ralph Baric??
NIH U01 AI069418: HIV/AIDS Clinical Trials Unit. Funded 2/01/2007-11/30/2013 (Coinvestigator). HHS N272200800005C: Vaccine and Treatment Evaluation Units. Funded 11/01/07- 10/31/14 (Co-Director), $2,494,361/yr.
NIH U01AI78407 : Clonal Analysis of the Human B Cell Response to HIV. Funded 2/01/08-01/31/13 (Co-Investigator), $150,000/yr (Emory component); $750,000 total.
NIH RO1 AI40338: Viral and Cellular Determinants of HIV-1 Assembly. Funded since 1994; (Principal Investigator). $250,000 initial period; $1,150,000 total- now transitioned to GM111027 (active, above).
NIH R01 CA27834: Genetics of Primate “D” Type Retroviruses. Funded 09/24/07- 11/30/12 (Co-investigator), $250,000/yr, $1,250,000 total.
NIH R01 AI084834: Defining Neutralization Breadth in HIV-positive serum. Funded 9/01/2009-8/31/2011 (Principal Investigator), $250,000 initial period, $500,000 total.
NIH R21 AI65312: Pseudovirion Formation by Live Vector HIV Vaccines. Funded 06/01/2006-05/31/2008 (Principal Investigator), $150,000 initial period; $300,000 total.
NIH R01 AI067101: Novel Assays for Inhibitors of HIV Assembly. Funded 6/15/2005- 5/31/2008 (Principal Investigator), $200,000 initial period; $550,000 total.
NIH U01 AI47985: HIV Vaccine Trials Units. Funded 06/00-05/05. $1,438,628 initial period; $7,637,877 total.
NIH P30 AI054999: Vanderbilt-Meharry Developmental CFAR. Funded 05/01/03-04/30- 06 (Co-investigator), $528,468 initial period; $1,633,442 total.
NIH R29 AI40338-01A1: Membrane Binding and Transport of HIV-1 Pr55Gag. Funded 03/97-03/2002 (Principal Investigator). $ 70,000/ year; $350,000 total.
NIH R21 AI44369 (Innovation Grant): Development of Enhanced HIV-1 Pseudovirion Vaccines. Funded 07/99-06/01(Principal Investigator). $140,000/ year; $260,000 total.
NIH R55 CA83527-01A1: Induction of KSHV replication by HIV-1. Funded 03/00-02/02 (Principal Investigator). $80,000 total.
.
.
.
NIH R01 AI52007: Development of Enhanced HIV-1 Pseudovirion Vaccines. Funded 06/2002-05/2007 (Principal Investigator), $225,000 initial period, $1,125,000 total.
NAI113678, GlaxoSmithKline: An open-label, multicenter, single arm study to evaluate the safety and tolerability of intravenous zanamavir in the treatment of hospitalized adult, adolescent, and pediatric subjects with confirmed influenza infection. Funded 10/02/12- 05/01/15. Principal Investigator.
P903-23, Cerexa: A multicenter, randomized, observer blinded, activ-controlled study to evaluate the safety, tolerability, efficacy, and pharmacokinetics of ceftaroline vs. comparator in pediatric subjects with acute bacterial skin and skin structure infections. Funded 01/01/2013-12/31/2014. Principal Investigator.
Merck Contract: Protocol 007: A Probe Study of the Safety, Tolerability, and Immunogenicity of a Three-dose Regimen of the Ad5 Gag Vaccine in Healthy Adults. Funded 04/01-12/02, $113,000 total (Principal Investigator).
Merck Contract: Protocol 012: A Probe Study of the Safety, tolerability, and Immunogenicity of the Ad5 HIV-1 Gag Vaccine. Funded 07/01/01-06/30/2003, $114,000 total (Principal Investigator).
Merck Contract: Protocol 016: A phase I dose-ranging study of the safety, tolerability, and immunogenicity of the Merck trivalent adenovirus serotype 5 HIV-1 gag/pol/nef vaccine in a prime-boost regimen in healthy adults. Funded 05/01/03-04/30/05, $117,000 total.
Basic Research Grant, Elizabeth Glaser Pediatric AIDS Foundation: Pseudovirion formation by live vector HIV vaccines. Funded 03/01/02-02/28/2004, $180,000 total.
.
.
LECTURESHIPS, SEMINAR INVITATIONS, AND VISITING PROFESSORSHIPS:
.
.
- Invited speaker, Peking University Department of Biomedical Engineering, Beijing, May 2015: “HIV-1 replication in macrophages”
- Invited speaker, Chinese Academy of Sciences, Institute of Biophysics, Beijing, May 2015: “Intracellular trafficking of the HIV envelope glycoprotein”
…………
Geeta K. Swamy, M.D.
Expertise: Infectious Diseases
Senior Associate Dean Vice Chair for Research & Faculty Development
Associate Professor, ObGyn
Department of Obstetrics & Gynecology, Division of Maternal-Fetal Medicine
Duke University, Durham, NC
2004 – 2006 Duke University Associate Faculty Department of Obstetrics & Gynecology Division of Maternal-Fetal Medicine & Division of Clinical & Epidemiological Research
2009 – present Duke University Vaccine Trials Unit Investigator Duke Translational Research Institute Durham, NC
2010 – 2018 Duke University Director Duke Perinatal Research Center Department of Obstetrics & Gynecology Division of Maternal-Fetal Medicine Durham, NC
2012 – present Duke University Associate Professor Department of Obstetrics & Gynecology Division of Maternal-Fetal Medicine & Durham, NC
2013 – present Duke University Human Vaccine Institute Investigator Durham, NC
2016 – 2017 Duke University Associate Dean for Regulatory Oversight & Research Initiatives in Clinical Research Durham, NC
Professional Awards and Special Recognition
2008 NIH Young Investigator Award Perinatal Research Society Meeting, Santa Fe, New Mexico
2010 NIH – NIAID Special Recognition for H1N1 pandemic
2013 and 2014 “Outstanding Reviewer” (Top 10%), Obstetrics and Gynecology
2014 “Outstanding Reviewer” for Vaccine
RESEARCH
Active Grants:
NICHD Maternal-Fetal Medicine Research Units (MFMU) 4/7/11 – 3/31/21
NIH-NICHD (Swamy) Principal Investigator Participation as a clinical site in the NICHD sponsored MFMU Research Network to investigate treatment strategies for common yet unresolved obstetric conditions through large multicenter collaborative trials
Past Grants:
Health Works for Women, NIH Summer Research Fellowship, University of North Carolina at Chapel Hill, Center for Health Promotion & Disease Prevention, 1994
PiiiTCH Study-Prevention of Influenza in Infants by Immunization of Their Household Contacts (CDC, Walter) Co-Investigator
NIH-NIAID (HHSN272200800057C, Swamy) Duke Site Principal Investigator
Randomized, Double-Blind Trial on Safety & Immunogenicity of Inactivated Trivalent Influenza Vaccine in Pregnant Women
NIH-NIAID (HHSN272200800057C, Swamy) Duke Site Principal Investigator A Phase II Study in Pregnant Women to Assess the Safety and Immunogenicity of an Unadjuvanted Sanofi Pasteur H1N1 Inactivated Influenza Vaccine Administered at Two Dose Levels
GlaxoSmithKline (Swamy) Cost-effectiveness of seasonal influenza vaccination during pregnancy An epidemiological study to develop and validate a model for estimating the costs and outcomes related to seasonal influenza vaccination during pregnancy for both mothers and infants through age 6 months.
Charles Hammond Research Fund (Gray) Mentor Assessing Decision Making & Acceptance of H1N1 Influenza Vaccine Administered in a Research Setting In Pregnancy
CDC-NCIRD – 1U01IP000190-01 (Swamy) Principal Investigator Effectiveness of a Vaccination Program in the Community ObGyn Setting The main objective of this 2-year project is to conduct a clinic-based study to develop and assess the effectiveness of a vaccination program for adolescent and adult women in the community Ob/Gyn setting.
ACOG/Merck & Company Research Award on Immunization (Fortner/Swamy) Mentor/Principal Investigator Compliance with Vaccination in the Obstetrical Setting with Novel H1N1 and Seasonal Influenza Retrospective review of births occurring in Durham, North Carolina during the 2009-2010 influenza season to evaluate influenza vaccination practices during the novel H1N1 pandemic.
Charles Hammond Research Fund (Swamy) Principal Investigator Association of Circulating Mitochondrial DNA Content and Preterm Birth Among Black Mothers
NIH-NIAID (HHSN272200800057C, Swamy) Duke Site Principal Investigator A Randomized, Double-Blind Trial on the Safety and Immunogenicity of Seasonal 2010-2011 Inactivated Trivalent Influenza Vaccine in Pregnant Women
.
.
Vaccine & Treatment Evaluation Units 9/16/13 – 9/15/23
NIAID (HHSN272201300017I, Walter and Swamy)
Co- Principal Investigator Participation as a clinical site in the NIAID sponsored VTEU Network to conduct clinical trials of vaccine and treatments for infectious diseases
Contract PI for the following active trials
A Phase I, Double-Blind, Dose Escalation Study to Evaluate the Safety and Pharmacokinetics of NTM-1632 vs Placebo Administered Intravenously in Healthy Adults
Group B Streptococcus (GBS) Colonization and Disease Among Pregnant Women: A Historical Cohort Study
A Phase I Cohort-Randomized, Double-Blind, Controlled Trial in Healthy Adults to Assess the Safety, Reactogenicity, and Immunogenicity of a Monovalent Inactivated Influenza A/H5N8 Virus Vaccine Administered Intramuscularly at Different Dosages Given With or Without AS03 or MF59 Adjuvants: Assessment of Immunological Responses and Lymphocyte Interplay
A Phase II, Double-Blind, Randomized, Placebo-Controlled, Multicenter Trial to Assess the Safety and Efficacy of 5% Monolaurin Vaginal Gel Administered Intravaginally for the Treatment of Bacterial Vaginosis
A Double Blind, Randomized, Placebo-Controlled, Phase I Dose Escalation Trial to Evaluate the Safety and Immunogenicity of an Inactivated West Nile Virus Vaccine, Hydro Vax-001, in Healthy Adults
An Opportunistic Study to Evaluate the Population Pharmacokinetics of Beta-lactam Antibacterials in Adults Including Elderly Subjects (POPS_SILVER)
A Population Pharmacokinetic Study to Evaluate Disposition of Azithromycin and Ertapenem in Pregnant Women Undergoing Cesarean Delivery After Failed Labor (POPS_CAN_DO)
Targeted Reduction of Antibiotics Using Procalcitonin in a Multi-center, Randomized, DoubleBlinded, Placebo-Controlled Non-Inferiority Study of Azithromycin Treatment in Outpatient Adults with Suspect Lower Respiratory Tract Infection (LRTI) and a Procalcitonin (PCT) Level of ≤0.25 ng/mL (TRAP-LRTI)
Phase 3, Randomized, Observer-Blind, Placebo-Controlled, Group-Sequential Study to Determine the Immunogenicity and Safety of a RSV F Nanoparticle Vaccine with Aluminum in Healthy 3rd Trimester Pregnant Women; and Safety and Efficacy of Maternally Transferred Antibodies in Preventing RSV Disease in their Infants Novavax, Inc. (Swamy) 12/1/15 – 7/31/19
Principal Investigator Clinical Immunization Safety Assessment (CISA) 9/29/15 – 9/28/18 Clinical Study of the Safety of Simultaneous Administration of Tetanus Toxoid, Reduced Diphtheria Toxoid and Acellular Pertussis Vaccine (Tdap) and Inactivated Influenza Vaccine (IIV) in Pregnant Women CDC (HHS200-2012-53663, Swamy) Principal Investigator
.
.
.
GlaxoSmithKline Speaker Services, 2009 – 2012
NIH-NIAID Division of Microbiology & Infectious Diseases Working Group on the Enrollment and Safety Assessments of Pregnant Women in Clinical Trials of Drugs and Vaccines, 2010 to 2015
National Vaccine Advisory Committee – Maternal Immunization Working Group – 2014 to 2016
Appointed Member, February 2017 to present
HPV Working Group, February – June 2018
Consultative Workshop on Immunology Research Gaps Related to Maternal Immunization – Bill & Melinda Gates Foundation, May 25-26, 2015
WHO Brighton Collaboration Global Alignment of Immunization Safety Assessment in Pregnancy (GAIA) – Chair, Fetal Distress Working Group – 2015
Independent Data Monitoring Committee, GlaxoSmithKline, Inc. – RSV vaccines for the protection of children, 2015 to 2017
Data Safety Monitoring Board, Randomized Controlled Trial of Influenza Vaccine and Meningococcal Vaccine in Pregnant Malian Women and Their Infants Up To 6 Months of Age, Sponsor: Bill & Melinda Gates Foundation, 2011-2016
………….
Gregg Sylvester, M.D., M.P.H. +
Expertise: Alternate Industry Representative
Vice President – Medical Affairs
Seqirus Inc., Summit, NJ
Physician | Public Health Expert
Pharmaceutical Executive Expert in vaccine preventable diseases, pediatrics and population health.
Career Highlights
• Head of Medical Affairs for Seqirus, a CSL company
• Launched Pfizer’s Pediatric and Adult Pneumococcal conjugate vaccine, as well as Meningococcal B vaccine in the USA
• Launched Merck’s HPV4 vaccine in over 100 countries, presented to numerous National Immunization Technical Advisory Groups (NITAGs), public health and medical societies
• Partnered with community organizations in Delaware to reduce infant mortality, teen pregnancy rates and HIV rates
Professional Experience
SEQIRUS, a CSL company Summit, N.J Vice President, Medical Affairs 2016 – present
• Responsible for the strategy and implementation of Medical Affairs plan
• Ensures appropriate use of Seqirus’ influenza vaccines
• Overseas Phase IV research and presents data to NITAGs and other key stakeholders.
PFIZER VACCINES Collegeville, Pa
Vice President, Medical and Scientific Affairs: Americas 2013 – 2016
• Spearheaded science-based rationale to preserve Prevnar 13 infant schedule in US recommendations
• Successfully achieved an adult Prevnar 13 recommendation from US Advisory Committee on Immunization Practice
• Accelerated launch of groundbreaking Meningococcal B vaccine to accommodate urgent public health need
Global Head of Medical Affairs for Pediatric Vaccines 2010 – 2013
• Global Medical Lead for Pfizer’s Pediatric vaccine, Prevnar 13
• Created medical strategy for Prevnar 13, an asset exceeding over $5 billion in revenue
• Created innovate systems to improved scientific exchanges in a complex, global environment
MERCK VACCINES West Point, PA
Global Head of Medical Affairs for Adolescent Vaccines 2005 – 2010
• Created the medical affairs strategy for Merck’s HPV4 vaccine, Gardasil
• Spokesperson for all Merck vaccines
• Traveled extensively throughout the world presenting to governmental officials, regulatory agencies and public health/medical congresses
CHRISTIANA CARE HEALTH SYSTEM Greenville, DE
Medical Director, Eugene DuPont Preventive Medicine & Rehabilitation Institute 2001 – 2005
• Led Preventive Medicine for Delaware’s largest health care organization
• Expanded the community-based health programs, serving more than 50,000 people/year
DELAWARE HEALTH & SOCIAL SERVICES New Castle, DE
Cabinet Secretary 1997 – 2001
• Reported directly to Governor of the State of Delaware
• Managed the largest state agency, with more than 5,000 employees and an operating budget of ~$1 billion
• Implemented Medicaid Managed Care and Children Health Insurance Program (sCHIP) in Delaware State
Health Officer 1995 – 1997
• Led Delaware’s Public Health Division
• Formulated Public Health policies and supervised programs addressing high infant mortality rates, teen pregnancy rates, and low childhood immunization rates
• Promoted to Cabinet Secretary within one year
Chief of Community Health & Director of Maternal & Child Health 1993 – 1995
• Directed the development and implementation of community-based public health programs for the state of Delaware
• Managed an annual budget of $30,000,000 and 330 public health professionals
Selected Board Positions
IMA World Health: Chairman of the Board: 2017-2018;
Board Member: 2013 – present
DONORS: The majority of IMA World Health’s projects are funded through grants from generous public funding agencies and foundations.
CORUS INTERNATIONAL
◦ IMA World Health | Corus World Health
◦ Lutheran World Relief
◦ CGA Technologies ==> https://www.cgatechnology.com/
◦ Ground Up Investing
◦ LWR Farmers Market Coffee
Selected Honors and Awards
Merck Global Human Health Awards – 2007 & 2006 Winner: Franchise of the Year; 2006 Winner: Best Support of International Markets and 2008, 2007 & 2006
Education & Training Fellowships:
- Public Policy – Joseph P. Kennedy, Jr. Foundation, assigned U.S. Senate, Washington, DC
- Epidemic Intelligence Service – Centers for Disease Control & Prevention, Atlanta, GA
- General Preventive Medicine Resident – Johns Hopkins School of Hygiene and Public Health, Baltimore, MD
- Pediatric Intern, Resident and Chief Resident – Children’s Hospital of Buffalo – State University of New York at Buffalo School of Medicine, Buffalo, NY
- Master of Public Health – Johns Hopkins School of Hygiene and Public Health, Baltimore,
- MD Doctor of Medicine – Albany Medical College, Albany, NY
- Bachelor of Arts – (History) – Ithaca College, Ithaca, NY
- Veteran Status Commissioned Officer, United States Public Health Service: Rank – Commander – 1990 -1993
Summary
These are the people who have no problem giving an unvetted vaccine to children and pregnant women before the safety data is available. After all, they have been doing it on a smaller scale most of their careers.
-Gail Combs
GC/wm (written/edited)