SPECIAL SECTION: Message For Our “Friends” In The Middle Kingdom
I normally save this for near the end, but…basically…up your shit-kicking barbarian asses. Yes, barbarian! It took a bunch of sailors in Western Asia to invent a real alphabet instead of badly drawn cartoons to write with. So much for your “civilization.”
Yeah, the WORLD noticed you had to borrow the Latin alphabet to make Pinyin. Like with every other idea you had to steal from us “Foreign Devils” since you rammed your heads up your asses five centuries ago, you sure managed to bastardize it badly in the process.
Have you stopped eating bats yet? Are you shit-kickers still sleeping with farm animals?
Or maybe even just had the slightest inkling of treating lives as something you don’t just casually dispose of?
中国是个混蛋 !!!
Zhōngguò shì gè hùndàn !!!
China is asshoe !!!
And here’s my response to barbarian “asshoes” like you:
OK, with that rant out of my system…
Justice Must Be Done.
The prior election must be acknowledged as fraudulent, and steps must be taken to prosecute the fraudsters and restore integrity to the system.
Lawyer Appeasement Section
OK now for the fine print.
This is the WQTH Daily Thread. You know the drill. There’s no Poltical correctness, but civility is a requirement. There are Important Guidelines, here, with an addendum on 20191110.
We have a new board – called The U Tree – where people can take each other to the woodshed without fear of censorship or moderation.
And remember Wheatie’s Rules:
1. No food fights
2. No running with scissors.
3. If you bring snacks, bring enough for everyone.
4. Zeroth rule of gun safety: Don’t let the government get your guns.
5. Rule one of gun safety: The gun is always loaded.
5a. If you actually want the gun to be loaded, like because you’re checking out a bump in the night, then it’s empty.
6. Rule two of gun safety: Never point the gun at anything you’re not willing to destroy.
7. Rule three: Keep your finger off the trigger until ready to fire.
8. Rule the fourth: Be sure of your target and what is behind it.
(Hmm a few extras seem to have crept in.)
Spot Prices
All prices are Kitco Ask, 3PM MT Friday (at that time the markets close for the weekend).
Last week:
Gold $1762.00
Silver $22.65
Platinum $981.00
Palladium $2000.00
Rhodium $14,050.00
This week, markets closed for the weekend at 3:00 PM Mountain Time
Gold $1758.00
Silver $22.75
Platinum $1031.00
Palladium $2167.00
Rhodium $14,850.00
Gold and Silver are holding steady…ridiculously so in fact. I read speculation that they’re going to bust out and surge. Why shouldn’t they? Inflation is galloping, the economy is headed for trouble once (some of) the companies out there actually stick to the jab mandate.
Platinum and palladium have taken decent jumps. Rhodium is up $800. That’s not too shabby either.
Personally? I’m liable to end up unemployed. I should buy a “I can’t afford to fix or replace this because of J** B*d*n” bumper sticker for my rear-ended car.
Part XXI: Nuclear Physics Uses The Hammer
Introduction
Last time I said that this time I’d take up stars. But I did some preliminary research on the history, and realized that we’re not quite there from a historical standpoint as our narrative is basically in the 1930s (except when I run ahead to finish something that started in the 1930s, like I did with neutrinos).
So I’m going to pick up the story of neutrons. Discovered in 1932 by James Chadwick, they turn out to be the “other” nucleon in the nucleus, supplementing protons. Similar in mass but with no electric charge, they were the actual occupants of the place in the nucleus that we had imagined held proton-electron pairs.
Because a neutron bears no electric charge, it has no trouble getting close to a nucleus and sticking to it, whereas a proton is repelled by any nucleus it approaches. If it can get close enough it will stick…but first it has to get close enough, and that’s a challenge. The same is true of alpha particles (which are bundles of two protons and two neutrons).
The “sticking” is provided by the strong nuclear force.
It’s as if you had two magnets, and were trying to bring the north poles close together. They push each other apart pretty hard, but if the magnets were covered with velcro, they’d stick together…once you overcame that repulsion.
Free neutrons are basically a new form of radiation, by the way; we have alpha and beta radiation (that bundle of four nucleons, and an electron, respectively), gamma radiation (a very high energy photon, X-rays on steroids), and now, we have free neutrons.
Free neutrons are scary. They’ll simply wander around until they find a nucleus to stick to…and they will more than likely make that nucleus radioactive. I don’t mind being around alpha and beta sources (so long as they’re not inside of me); they’re trivial to shield against. Gamma rays are intimidating because they penetrate very thick shielding. All three of these, if they get to you, will blast some chemical bond to smithereens which can either mean nothing or cause big problems, depending on what it was they hit. They won’t make you radioactive. But the neutrons just sort of wander aimlessly through matter, unaffected by very much until they find a nucleus–and nuclei don’t take up much space, in fact they take up virtually none of it. Whatever nucleus they hit becomes a new (and likely radioactive) isotope.
That nucleus, with the extra neutron, may find itself with “too many” neutrons, and one of the neutrons will then change into a proton, via the weak force. This has the effect of making that atom a different element, the one next over to the right on your handy-dandy periodic table. That increases the atomic number, Z, by 1, while leaving the mass number (the total number of protons and neutrons) the same.
OK, that’s the end of the review. Now on with the story, which is complicated. I apologize in advance if this is completely un-followable. And if I somehow managed to garble it in trying to simplify it, I apologize for that as well. [Most of this is from the Wikipedia article on Lise Meitner, and the article on the discovery of fission.]
Transmutation
Nuclear physicists had all kinds of fun playing with neutrons through the 1930s (and beyond). Enrico Fermi, in Rome, made a hobby of bombarding different elements with neutrons to see what would happen; first creating a more neutron rich isotope of the starting element, then monitoring the beta decay, determining half lives and energies, which are different for each isotope. Sometimes there’d be multiple decays, because one wasn’t enough to get to a stable isotope.
Remember, each such beta decay moves you one to the right, to the next higher atomic number. This led to an irresistibly tantalizing question.
What happens if you pick the element with the highest atomic number, uranium with Z=92, and bombard it with neutrons?
Shouldn’t you get element 93, previously utterly unknown, in fact, previously nonexistent?
Fermi tried it. And he got a whole bunch of different kinds of beta radiation out of it. He concluded that he had created a “transuranic” element. Not so fast though. Aristid von Grosse suggested that what Fermi had found was a new isotope of protactinium (element 91, not 93). This “wait a minute” wasn’t enough to prevent Fermi’s winning the 1938 Nobel Prize for Physics for this work, not just with uranium but the other elements as well.
But there was enough controversy that someone needed to dig in and figure out if we were looking at element 93 or protactinium.
And who better to do that than Lise Meitner and Otto Hahn, the discoverers of protactinium? Their collaboration at Kaiser Wilhelm institute in Berlin had lapsed, but this question got the two of them back together. From 1934-1938 the two of them, along with Otto Frisch, dug into the matter.
Initially, Meitner and Hahn believed they had created elements 93, 94, 95 and even 96. But as time went on Meitner became less certain.
Part of the muddle came from the fact that it was wrongly believed that only the lanthanide elements had that special row at the bottom of the table, pulled out from the main body so it would fit nicely on a landscape piece of paper. Actinium was placed two spots below yttrium, thorium below hafnium, protactinium below tantalum and uranium below tungsten (or as the Germans called it, “wolfram”). Indeed the chemical behavior of these elements could be a bit confusing, but it would eventually turn out that that stopped with element 93, which behaved more like a lanthanide. That whole sequence of elements in fact belonged in a second footnote row below the lanthanides.
For example, Fermi had found a rhenium-like element in his experiments and, in the belief that element 93 was directly below rhenium in the periodic table, concluded that that is what he had found. (In fact, he had found technetium, the then-undiscovered element above rhenium in the table, and didn’t realize it–but I’m getting ahead of myself here.)
This mistaken belief, at the time, bunged up any attempt to chemically analyze the products of the neutron bombardment. When element 93 is expected to behave like rhenium, for instance, rather than like a rare earth, it’s kind of difficult to figure out what’s going on.
One thing Meitner and Hahn wer fairly confident of: when they bombarded uranium, which was mostly uranium-238 (92 protons and 146 neutrons), they were indeed getting, as step one, uranium-239, with a 23 minute half life. They were able to do chemistry on it and prove that it was, indeed uranium.
After that it was a muddle. There seemed to be three different reactions, all from uranium-239, one with a ten second half life, one with a twenty second half life, and one with a 23 minute half life.
In 1937 Meitner and Hahn each published a report. Hahn was emphatic that they had found transuranic elements (“Above all, their chemical distinction from all previously known elements needs no further discussion”); Meitner was pretty certain almost everything was a product of uranium-238, somehow, but figured the three most prominent products were isomers.
Er, what’s an isomer?
As if it isn’t difficult enough to recall that elements come in isotopes, with the same number of protons but different number of neutrons, it turns out that some of the isotopes themselves come in different forms, some more energetic than others, and that the more energetic form eventually just blasts out pure energy (a gamma ray photon) and settles down to become the less energetic, and (usually) more stable form, having kept all of its protons and neutrons intact (but, likely, having dropped mass a bit). An isotope like this gets an “m” after the number.
For example, consider protactinium-234m, which has a 1.17 minute half life, and ejects a photon as it settles down to become protactinium-234, with a half life of 6.70 hours. When Pa-234m was discovered in 1913, we weren’t clear on the concept of isotopes, so it was considered a new element and named brevium for its brief half life.
When “regular” Pa-234 was discovered in 1921, that marked the discovery of nuclear isomers; it was the first such distinction between an “m” isotope and a “regular” isotope. And, interestingly, the discoverer was Otto Hahn, who later on in 1937 found his colleague using the concept to argue against his interpretation of the U-239 decay products!
[Side note: Probably the most useful isomer today is technetium-99m. It’s a decay product of molybdenum-99, which has about a 30 hour half life. Mo-99 is sent to hospitals, which extract the Tc-99m chemically, embed it in larger molecules, perhaps favored by muscles, then inject that into patients and watch where the gamma rays come from. This can be used to diagnose heart problems, though it does mean the patient is a source of gamma rays for a while. Tc-99m has a six hour half life, after which it blasts out a fairly weak gamma ray and settles down to Tc-99, which has a much longer half life (hundreds of thousands of years) and will ultimately beta decay and become ruthenium-99. The patient generally gets rid of the technetium-99 within days, so no digging up bodies to try to get the ruthenium, please.]
Meitner concluded her report with the following: “The process must be neutron capture by uranium-238, which leads to three isomeric nuclei of uranium-239. This result is very difficult to reconcile with current concepts of the nucleus.”
Another group in Paris decided to investigate as well. They ultimately found a product that was chemically very similar to lanthanum (element 57). (It turned out it couldn’t be more similar, as it was lanthanum, but I get ahead of myself again.)
Did I just almost forget to mention Meitner was Jewish?
What does that matter? Normally it wouldn’t matter in the slightest, but in mid 1930s Berlin, it mattered a great deal. And it was mattering more and more as time passed.
Meitner Has To Flee
Meitner had been kept safe, somewhat, by the fact that she was an Austrian, but on March 12, 1938, Austria was annexed by Germany. Her Austrian citizenship was moot as there was no Austria to be a citizen of. Niels Bohr and Paul Scherrer invited her to take positions in Denmark and Switzerland, respectively, but Carl Bosch at KWI said she could remain. By May, though, Meitner learned that her situation was being looked at by the no-doubt misnamed Reich Ministry of Science, Education and Culture.
Although many people outside of Germany wanted to give her refuge, there were all sorts of bureaucratic snafus. For instance, she couldn’t go to Denmark no matter how much Niels Bohr wanted her there, because Denmark considered Austrian passports to be invalid. Germany also forbade academics to leave the country.
By July the situation was critical. Dirk Coster, a Dutch scientist, convinced the Netherlands to accept Meitner, and on July 12, she showed up for work at KWI as usual, staying late to mark up an associate’s paper for publication. The next day she and Coster took a train on a lightly used rail line to the Dutch border. Otto Hahn had given her his mother’s ring and “Frau Professor” was apparently thought to be the wife of the Dutch professor, so the German border guards didn’t stop her. She got out, with ten marks and her summer clothes, and the ring she could sell for money if needed. (The story is much more complex, and given in the Wikipedia article on Meitner.)
Once Meitner was safely out of Nazi Germany, work continued long-distance. Hahn and Strassman at KWI decided to try to replicate the Paris group’s results, and found what they thought was radium (element 88).
Figuring that the neutron hitting uranium-238 was creating uranium-239, which then gave up two alpha particles to become radium-231, they dug a little more carefully, and decided to extract the radium from the sample.
Radium lies directly under barium (element 56) on the periodic table (it was properly understood back then, unlike uranium), and the two elements have an affinity with each other. If there was any radium in the products, barium could be used to draw it out, then it could be separated from the barium without interference from all the other “stuff” in the sample.
Indeed, the barium came out radioactive, indicating that there was radium in it. So it looked like they had found their radium, and the two alpha decays.
But then they couldn’t separate the radium from the barium.
The extraction process used was tested by putting known samples of radium into the barium, and they were separated out without any trouble.
The Light Dawns
Finally they were forced to conclude that the reason they couldn’t find any radium in the barium, is that it was barium.
A radioactive isotope of element 56 was coming out of uranium-239.
Meitner and Frisch finally realized that what was happening. They had gotten together for Christmas in 1838, and were out cross-country skiing having a rather atypical conversation.
What if, they thought, the uranium nucleus were simply splitting? The prevailing model of the nucleus was called the “liquid drop” model, treating it as similar to a drop of liquid; if it were under enough tension that it wanted to break up, a neutron could add just enough “oomph” to happen, just like a very large drop of water wants to split into smaller drops. (Incidentally the liquid drop model, though not the most advanced model, is still good enough to be of some use today.)
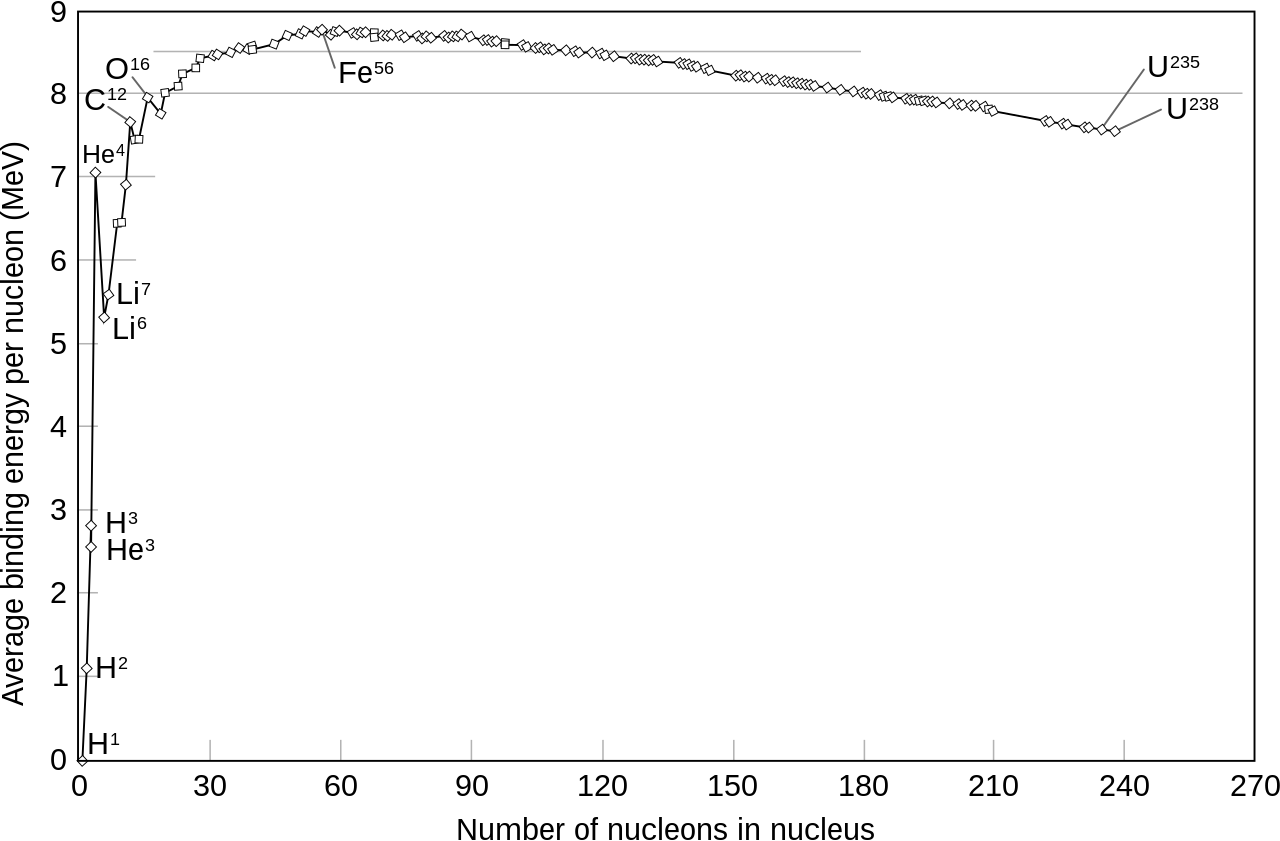
However the two pieces would find themselves outside the range of the strong nuclear force, and repel each other quite forcefully. About 200 million electron volts–about a fifth the mass/energy of a proton–would be released as the two pieces flew apart. Where would it come from?
Meitner was able to figure out that the two pieces’ binding energy was high enough compared to the uranium’s binding energy that the 200 MeV would be supplied by that.
It fit.
Nuclear fission was real.
Uranium could be induced to split and release a lot of energy. The lanthanum, technetium and barium were real. It just depended on exactly how the split happened, which particular smaller elements you’d get.
When Frisch told Niels Bohr of this, Bohr literally smacked his own forehead and exclaimed, “What idiots we have been!”
Fermi was also embarrassed; that part of his work bombarding things with neutrons that had to do with uranium turned out to be misinterpreted, and the 1938 Nobel prize he had just been told he would receive was in part awarded for his transuranium “discoveries.” Just in time though; he added a footnote to his acceptance speech to explain what they had just figured out.
In the fullness of time, it developed that those 10 and 20 second reactions Meitner, Hahn and Frisch were seeing were fission products. But the 23 minute reaction really was a decay into element 93, isotope 239.
And it was the small amount of uranium-235 that was fissioning, not the uranium-238.
And we now had a new form of radioactive decay: fission, spontaneous fission. Uranium 236’s most common decay mode is this.
The Bomb
The rest of the story is much more famous, though at the time it was shrouded in secrecy. The US government, alerted by none other than Albert Einstein’s letter to FDR concerning the potential of such massive releases of energy, created the Manhattan Project to build a nuclear bomb.
Much of this early research had been done in Nazi Germany. What if they, too, were working on The Bomb?
It turns out that when uranium-235 is hit by a fairly slow moving neutron, it becomes, for just an instant, uranium-236, which is what fissions into two large pieces. But there are also two or three bare neutrons released; if they can be slowed down and then induced to hit more uranium-235, you can have a chain reaction, each step doubling or even tripling the energy release as more and more uranium-235 catches neutrons and releases yet more neutrons.
However, you need a fairly substantial amount of U-235 for this to work. If it’s a small lump of the stuff, the freed neutrons will probably exit the sample before they find a nucleus to hit. There’s a critical mass that must be brought together for the chain reaction to take off.
The bigger difficulty, of course was that uranium-235 is only a small fraction, less than one percent, of uranium.
So one of the two approaches taken was to try to extract “enriched” uranium-235 from uranium by reacting the uranium with fluorine to create uranium hexafluoride gas, which could then be centrifuged to separate out the slightly lighter U-235. This work was done at Oak Ridge, Tennessee. Once you have the enriched uranium-235, it’s dead easy to make a bomb. Bring two small masses together, enough to make a critical mass, make sure there’s a neutron source nearby, and, KABOOM!!
The other approach involved those transuranics. Uranium-239 was beta decaying into neptunium-239; neptunium 239 was in turn beta decaying into plutonium-239. The two new elements were named to continue the series. Uranium had been named after the planet uranus; the next two elements were named after neptune and pluto (then believed to be a planet).
And it turns out that plutonium-239 is easy to produce; just bombard regular uranium with neutrons–and it too will fission when struck by a neutron. The trick is to get enough of it together close enough that the excess neutrons will find another plutonium-239 atom before exiting the mass. As it happens, Pu-239 must be compressed for it to work. And getting that to happen precisely right was a challenge.
It’s a good thing that the easy-to-make bomb requires the hard-to-make material, and the easy to make material is hard to make a bomb out of.
But we did both.
The U-235 bomb was deemed so simple it wouldn’t need a test. Thus it was dropped on Hiroshima on August 6, 1945 (Germany had surrendered in May of that year, after millions had given their lives to put the mad dictator Hitler down). It worked beautifully, releasing energy equivalent to about 20 thousand tons of TNT, all at once.
The Pu-239 bomb, however, was the first nuclear detonation. It was tested at Trinity site in New Mexico on July 16th, then a second example was dropped on Nagasaki on August 9th.
The bombs killed tens of thousands of people, but likely saved at least ten times as many lives. Had the United States needed to invade the Japanese home islands, there likely would have been two million casualties.
The neutron had gone from being an abstract thing cared about only by some physicists trying to figure out what keeps atoms together…to something as impossible to ignore as a slap across the face.
And this wasn’t even the end of that road.
Obligatory PSAs and Reminders
China is Lower than Whale Shit
Remember Hong Kong!!!
中国是个混蛋 !!!
Zhōngguò shì gè hùndàn !!!
China is asshoe !!!
China is in the White House
Since Wednesday, January 20 at Noon EST, the bought-and-paid for His Fraudulency Joseph Biden has been in the White House. It’s as good as having China in the Oval Office.
Joe Biden is Asshoe
China is in the White House, because Joe Biden is in the White House, and Joe Biden is identically equal to China. China is Asshoe. Therefore, Joe Biden is Asshoe.
But of course the much more important thing to realize:
Joe Biden Didn’t Win
乔*拜登没赢 !!!
Qiáo Bài dēng méi yíng !!!
Joe Biden didn’t win !!!




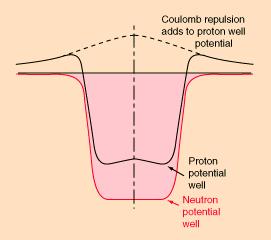
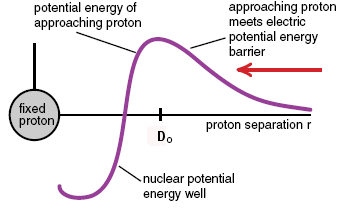 Imagine a proton coming in from the side, towards the nucleus (not shown) at center. It has to have enough velocity to travel over the “coulomb barrier” (repulsion from electrostatic forces), after which it can drop into the well because it is attracted by the nuclear force. This is actually a very good analogy because gravitational potential barriers are actual hills you’d have to be able to coast over. This one is a combination of the electrostatic and nuclear forces as they act on protons. In red is shown the situation for neutrons, which only respond to the nuclear force.
Imagine a proton coming in from the side, towards the nucleus (not shown) at center. It has to have enough velocity to travel over the “coulomb barrier” (repulsion from electrostatic forces), after which it can drop into the well because it is attracted by the nuclear force. This is actually a very good analogy because gravitational potential barriers are actual hills you’d have to be able to coast over. This one is a combination of the electrostatic and nuclear forces as they act on protons. In red is shown the situation for neutrons, which only respond to the nuclear force.